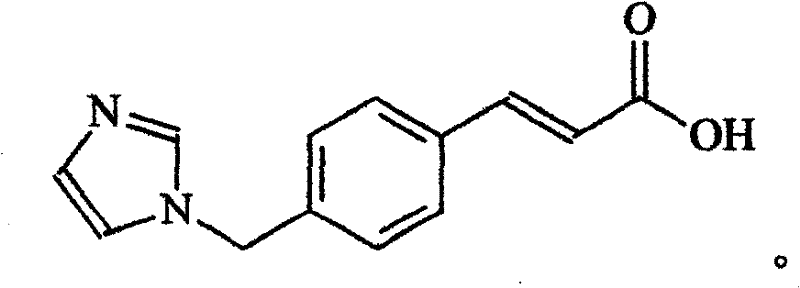
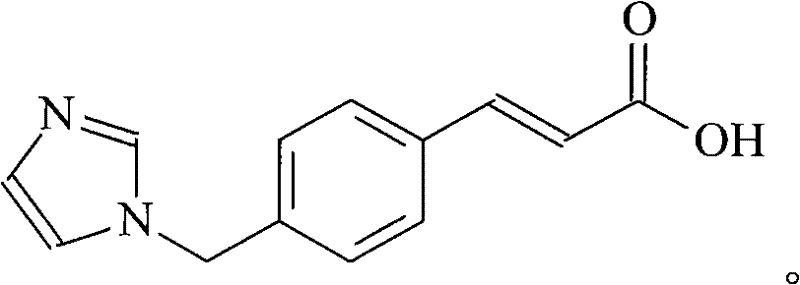
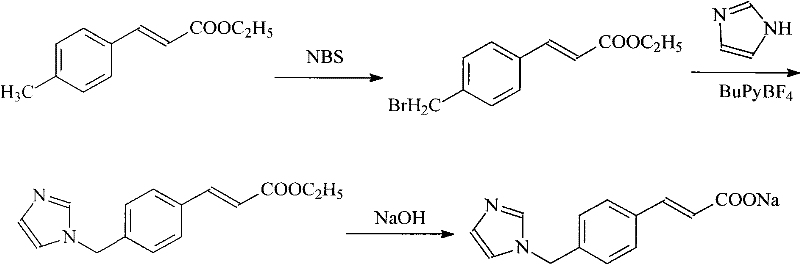
High-purity ozagrel compound
A high-purity compound technology, applied in the field of medicine, can solve the problems of low purity of sodium ozagrel, high cost, unsatisfactory foreign matter, etc., and achieve the effects of being suitable for large-scale industrial production, easy to operate, and simple in method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The refining of embodiment 1 ozagrel
[0021] 100g of crude ozagrel was added into 2000ml of water to form an aqueous dispersion, and then 0.1mol / L sodium hydroxide solution was added dropwise to the aqueous dispersion until clarified to obtain a clear solution, and 23g of activated carbon was added to the resulting clear solution for adsorption for 20 minutes. Filtrate decarburization, add 0.5 mol / L hydrochloric acid solution to the obtained filtrate to adjust the pH to 1.0, precipitate a solid, filter, wash with 50ml×3 water, and dry under reduced pressure at 50°C for 8 hours to obtain high-purity ozagrel 92.6 g, the yield is 92.6%, and the purity detected by HPLC is 99.94%.
Embodiment 2
[0022] The refining of embodiment 2 ozagrel
[0023] Add 100 g of crude ozagrel into 2000 ml of water to form an aqueous dispersion, then add dropwise 0.5 mol / L sodium hydroxide solution to the aqueous dispersion until clarification, to obtain a clear solution, add 1.15 g of activated carbon to the resulting clear solution for adsorption for 30 min, Filtrate decarburization, add 1 mol / L nitric acid solution to the obtained filtrate to adjust the pH value to 3.0, precipitate a solid, filter, wash with 50ml×3 water, and dry under reduced pressure at 60°C for 5 hours to obtain 93.1g of high-purity ozagrel , the yield was 93.1%, and the purity detected by HPLC was 99.92%.
Embodiment 3
[0024] The refining of embodiment 3 ozagrel
[0025] Add 100 g of crude ozagrel into 2000 ml of water to form an aqueous dispersion, then add dropwise 0.2 mol / L sodium hydroxide solution to the aqueous dispersion until clarification, to obtain a clear solution, add 2.3 g of activated carbon to the resulting clear solution for adsorption for 30 min, Filtrate decarburization, add 5 mol / L phosphoric acid solution to the obtained filtrate to adjust the pH value to 2.0, precipitate a solid, filter, wash with water 50ml×3, and dry under reduced pressure at 55°C for 6 hours to obtain 94.5g of high-purity ozagrel , the yield was 94.5%, and the purity detected by HPLC was 99.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com