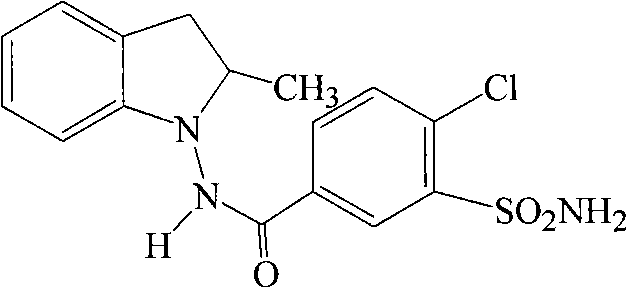
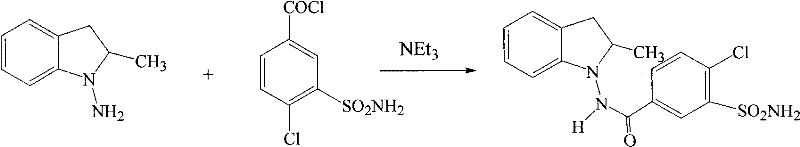
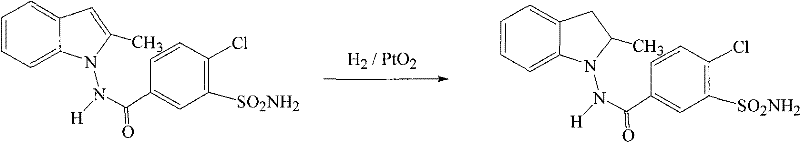Method for synthesizing indapamide
A technology for the synthesis of indapamide and its synthesis method, which is applied in the field of synthesis of the diuretic and antihypertensive drug indapamide, achieving the effects of high yield, good reaction selectivity, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a 1000mL reaction flask, add ethyl acetate (300ml), N-amino-2-methylindoline (14.8g, 0.1mol), 4-chloro-3-sulfamoylbenzoic acid (23.6g, 0.1 mol), chlorinated 1,3-dimethyl-2-chloroimidazoline (11.4 grams, 0.1 mol), stirred at 15°C for 30 minutes, added dropwise triethylamine (10.1 grams, 0.1 mol), and then React at room temperature for 12 hours. Water (60 mL) was added, filtered and dried to give crude indapamide. Through isopropanol-water recrystallization, 32.7 g of a white crystalline product was obtained with a yield of 89.4%, an HPLC purity of 99.67%, and a melting point of 165-167°C. The infrared absorption spectrum and nuclear magnetic resonance absorption spectrum were consistent with those reported in literature.
Embodiment 2
[0036] In a 1000mL reaction flask, add tetrahydrofuran (400ml), N-amino-2-methylindoline hydrochloride (18.5g, 0.1mol), 4-chloro-3-sulfamoylbenzoic acid (28.3g , 0.12 mol), chlorinated 1,3-dimethyl-2-chloroimidazoline (14.8 g, 0.13 mol), stirred at 10°C for 30 minutes, added dropwise to pyridine (11.8 g, 0.15 mol), and then at room temperature React for 16 hours. Water (80 mL) was added, filtered and dried to give crude indapamide. Through ethanol recrystallization, 34 grams of white crystalline product was obtained, with a yield of 93.1%, an HPLC purity of 99.72%, and a melting point of 165-167°C.
Embodiment 3
[0038] In a 1000mL reaction flask, add 1,2-dichloroethane (300ml), N-amino-2-methylindoline methanesulfonate (24.4g, 0.1mol), 4-chloro-3- Sulfamoylbenzoic acid (28.3 grams, 0.12 moles), chlorinated 1,3-dimethyl-2-chloroimidazoline (14.8 grams, 0.13 moles), was stirred at 15°C for 30 minutes, and triethylamine ( 15.2 g, 0.15 mol), and reacted at room temperature for 15 hours. Water (100 mL) was added, filtered and dried to give crude indapamide. Through ethanol recrystallization, 32.4 g of white crystalline product was obtained, with a yield of 88.5%, an HPLC purity of 99.69%, and a melting point of 165-167°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com