Method for producing 2-alkyl-2H-(halo)isoquinoline-1-ketone
An isoquinoline and alkyl technology, applied in the production field of isoquinoline derivatives, can solve the problems of low yield, complicated operation, etc., and achieve the effects of high purity, wide sources, and large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
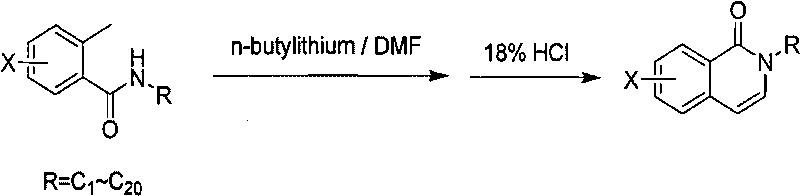
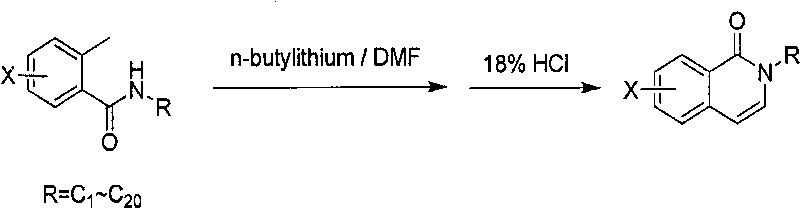
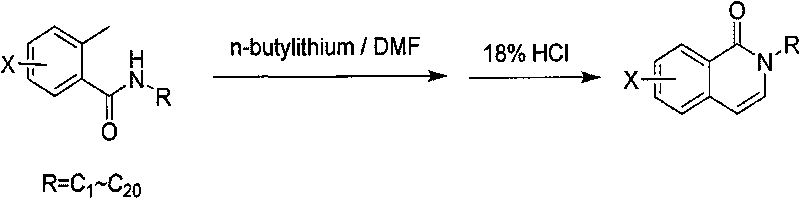
[0013] The method for producing 2-alkyl-2H-(halogenated) isoquinolin-1-ones, the specific reaction formula is as follows:
[0014]
[0015] Benzoylalkylamine (207g, 1.17mol) was dissolved in 650ml of anhydrous tetrahydrofuran, cooled to 0°C, 0.5L of 2.5M n-butyllithium solution was added dropwise, the temperature was maintained at 5°C, and stirred for 1 hour. At 15°C, start to add 97ml of N,N-dimethylformamide dropwise, maintain the temperature at 0-9°C, after the dropwise addition is completed. After the addition was complete, allow to gradually warm to room temperature and stir for 1 hour. At 0-10°C, add 400ml of 18% aqueous hydrochloric acid solution dropwise, keep the temperature not exceeding 30°C, stir for about 0.5-1 hour, separate the liquids, take the organic phase, dry over magnesium sulfate, and concentrate to obtain the crude product. Recrystallize from petroleum ether / ethyl acetate to obtain a white solid. Yield 25%, weighing 50g, purity 90%.
Embodiment 2
[0017] Benzoylalkylamine (207g, 1.17mol) was dissolved in 650ml of anhydrous tetrahydrofuran, cooled to 10°C, 1L of 2.5M n-butyllithium solution was added dropwise, the temperature was maintained at 0°C, and stirred for 1 hour. At 0°C, 146ml of N,N-dimethylformamide was started to be added dropwise, and the temperature was maintained at 0°C. After the dropwise addition was completed. After the addition was complete, allow to gradually warm to room temperature and stir for 1 hour. At 5°C, add 451ml of 18% hydrochloric acid solution dropwise, keep the temperature not exceeding 30°C, stir for about 1 hour, separate the liquids, take the organic phase, dry over magnesium sulfate, and concentrate to obtain a crude product. Recrystallize from petroleum ether / ethyl acetate to obtain a white solid. Yield 51%, weighing 100g, purity 99.9%.
Embodiment 3
[0019] Benzoylalkylamine (207g, 1.17mol) was dissolved in 650ml of anhydrous tetrahydrofuran, cooled to 0°C, and 1L of 2.5M n-butyllithium solution was added dropwise, maintaining the temperature at 5°C, and stirred for 1 hour. At 30°C, 146ml of N,N-dimethylformamide was added dropwise, and the temperature was maintained at 9°C. After the dropwise addition was completed. After the addition was complete, allow to gradually warm to room temperature and stir for 1 hour. At 0-10°C, add 250ml of 18% hydrochloric acid solution dropwise, keep the temperature not exceeding 30°C, stir for about 1 hour, separate the liquids, take the organic phase, dry over magnesium sulfate, and concentrate to obtain a crude product. Recrystallize from petroleum ether / ethyl acetate to obtain a white solid. Yield 45%, weighing 88g, purity 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com