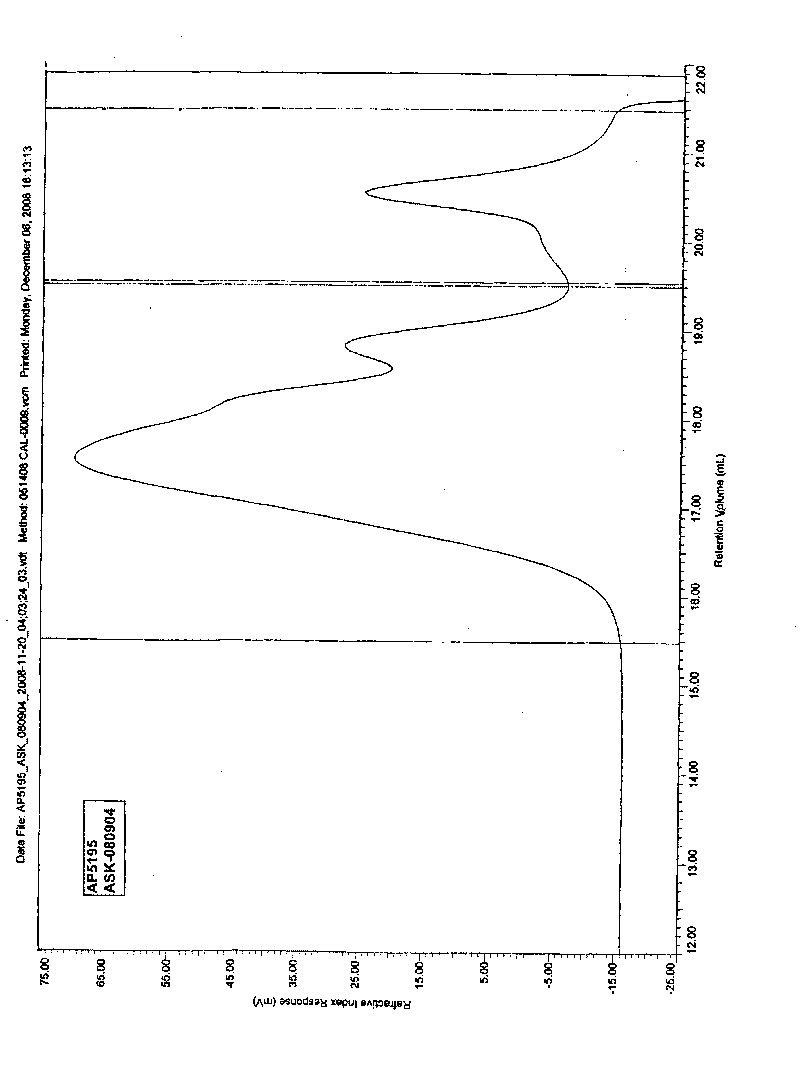
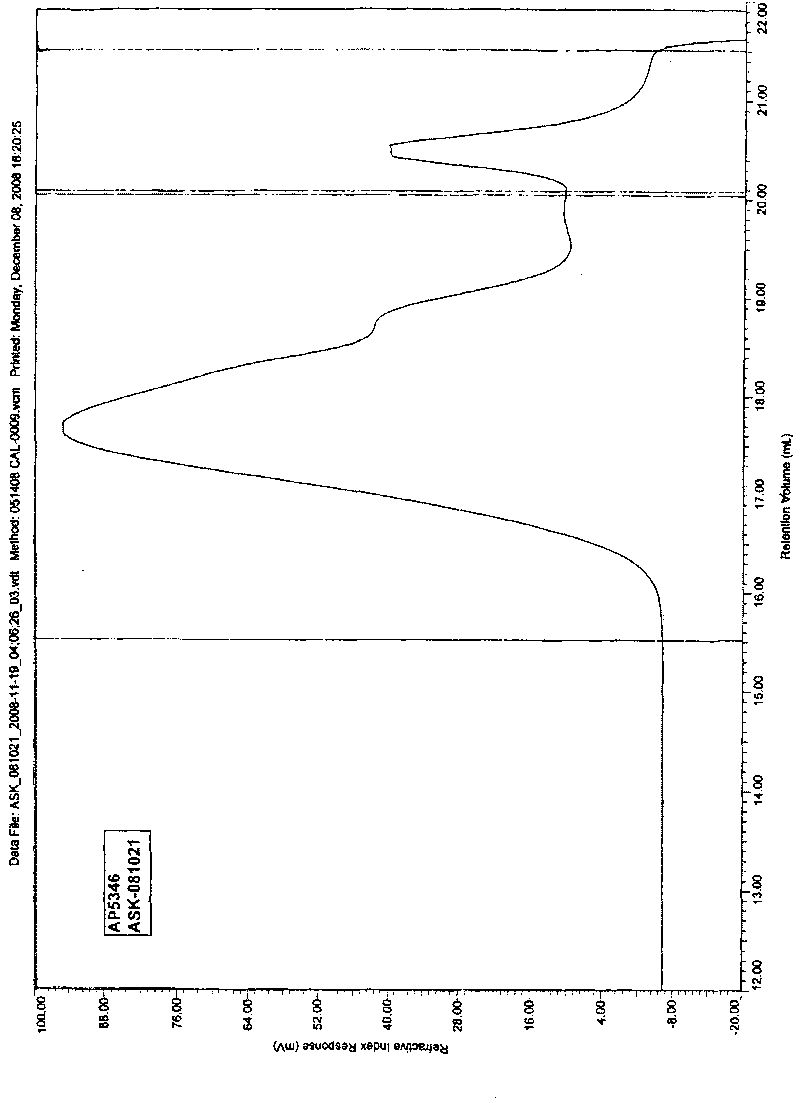
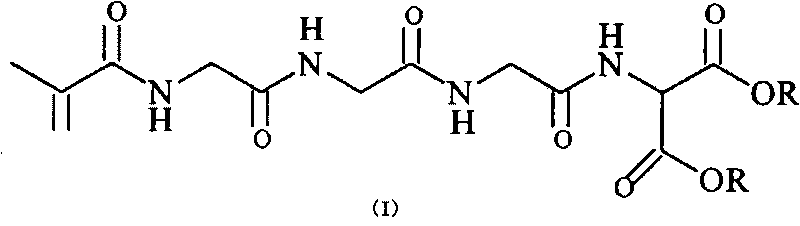
2-(2-methacrylamide triglycyl) aminomalonate ester as well as preparation method and application thereof
A technology of methacrylamidotriglycyl and aminomalonate is applied in chemical instruments and methods, organic chemistry, peptides, etc., and can solve the problems of low total yield, high cost, complicated synthesis method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Preparation of Diethyl 2-(2-Methacrylamidoglycylglycylglycylglycyl)aminomalonate
[0084] (1) Preparation of 2-(Fmoc-glycylglycylglycyl)aminomalonate diethyl ester
[0085] Drop into diethyl aminomalonate hydrochloride 21.2g (0.1mol) and 40ml deionized water in the reaction flask, slowly add 11.5 grams (0.14mol) sodium bicarbonate powder after stirring and dissolving, a large amount of bubbles are released, add After completion, the reaction was stirred for 20 minutes, extracted with ethyl acetate (50ml*3), the organic layers were combined, dried, filtered to remove the desiccant, and concentrated under reduced pressure to remove the solvent to obtain diethyl aminomalonate (for use).
[0086] Put 2g of 1-hydroxybenzotriazole (HOBT) (0.015mol), 800ml of acetonitrile, pre-prepared diethyl aminomalonate (0.1mol) and 37 grams of Fmoc-glycylglycine into another reaction flask Acylglycine (0.09mol), under nitrogen protection, cooled to about 0°C, added 20.6g of dicyclohexylc...
Embodiment 2
[0103] Preparation of Diethyl 2-(2-Methacrylamidoglycylglycylglycylglycyl)aminomalonate
[0104] (1) Preparation of 2-(Boc-glycylglycylglycyl)aminomalonate diethyl ester
[0105] Drop into diethyl aminomalonate hydrochloride 318g (1.5mol) and 700ml deionized water in the reaction flask, slowly add 161 grams (1.92mol) sodium bicarbonate powder under stirring, stir and react for 20 minutes after adding, Extract with ethyl acetate (800ml*3), combine the organic layers, dry, filter to remove the desiccant, and concentrate under reduced pressure to remove the solvent to obtain diethyl aminomalonate.
[0106] Put 30 g of 1-hydroxybenzotriazole (HOBT) (0.22 mol), 12 L of acetonitrile, pre-prepared diethyl aminomalonate (1.5 mol) and 389 g of Boc-glycylglycyl in a reaction kettle Glycine (1.35mol), under nitrogen protection, cooled to about 0°C, added 309g of dicyclohexylcarbodiimide (1.5mol) in portions, and controlled the temperature below 5°C. After the addition, it was reacted a...
Embodiment 3
[0123] Preparation of dimethyl 2-(2-methacrylamidoglycylglycylglycylglycyl)aminomalonate
[0124] (1) Preparation of dimethyl 2-(Fmoc-glycylglycylglycyl)aminomalonate
[0125] Drop into dimethyl aminomalonate hydrochloride 14.7g (0.1mol) and 40ml deionized water in the reaction bottle, slowly add 12.6 grams (0.15mol) sodium bicarbonate powder after stirring and dissolving, a large amount of bubbles are released, add After completion, the reaction was stirred for 20 minutes, extracted with ethyl acetate (50ml*3), the organic layers were combined, dried, filtered to remove the desiccant, and concentrated under reduced pressure to remove the solvent to obtain dimethyl aminomalonate (for use).
[0126] Put 1.35g of 1-hydroxybenzotriazole (HOBT) (0.01mol), 800ml of acetonitrile, dimethyl aminomalonate (0.1mol) and 30.8 grams of Fmoc-glycylglycylglycine into another reaction flask (0.075mol), under nitrogen protection, cooled to about 0°C and added 20.6g of dicyclohexylcarbodiimide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com