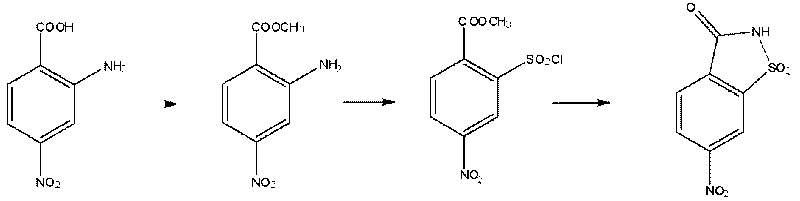
Preparation method of 6-nitrosaccharin
A technology of nitrosaccharin and nitrobenzoic acid, applied in the field of preparation of 6-nitrosaccharin, can solve the problems of difficulty in industrialization, serious environmental pollution, difficult control, etc., and achieves low price, no environmental pollution and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation method of 6-nitrosaccharin of this embodiment has the following steps:
[0020] ①Preparation of methyl 2-amino-4-nitrobenzoate: Add 40g of 2-amino-4-nitrobenzoic acid and 300ml of methanol (237g) into a 500ml four-neck flask as the reactants, and add 54g of Thionyl chloride is used as an acid catalyst to generate 2-amino-4-nitrobenzoic acid methyl ester. The reaction slowly exotherms to boiling, and the reaction is heated and refluxed for 10 hours after dropping. After the reaction, vacuum was applied under reduced pressure to evaporate the methanol, and the methanol was recovered. The remaining concentrate was dissolved by adding 300 ml of ethyl acetate, and the resulting mixture was washed with a saturated aqueous sodium bicarbonate solution until it became neutral. Heat the entire system to evaporate the water, continue heating to evaporate the ethyl acetate and concentrate the organic phase to make the product precipitate. After recovering most of the e...
Embodiment 2
[0025] The preparation methods of Example 2 to Example 3 are basically the same as that of Example 1, except that the acidic catalyst used in step ① is different. The acidic catalyst used in Example 2 is concentrated sulfuric acid (98wt%). Example 3 The acid catalyst used is gaseous hydrogen chloride. The method for adding concentrated sulfuric acid in Example 2 is dropwise addition, and the method for adding gaseous hydrogen chloride in Example 3 is to pass the gaseous hydrogen chloride into the reaction system. See Table 1 for details.
[0026] Table 1
[0027]
Embodiment 4 to Embodiment 8
[0029] The preparation methods of Example 4 to Example 8 are basically the same as that of Example 1, except that the acidic medium and diazotization temperature used in step ② are different. See Table 2 for details.
[0030] Table 2
[0031]
[0032] It can be seen from Table 2 that the yield at the reaction temperature of 10°C to 15°C is higher than other temperatures. On the other hand, the acidic medium is different and the reaction effect is also different. It is better to use hydrochloric acid as the reaction medium than other reaction media.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com