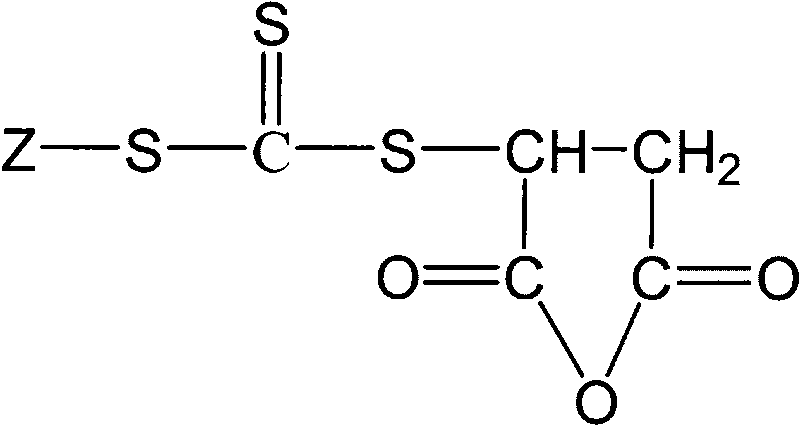
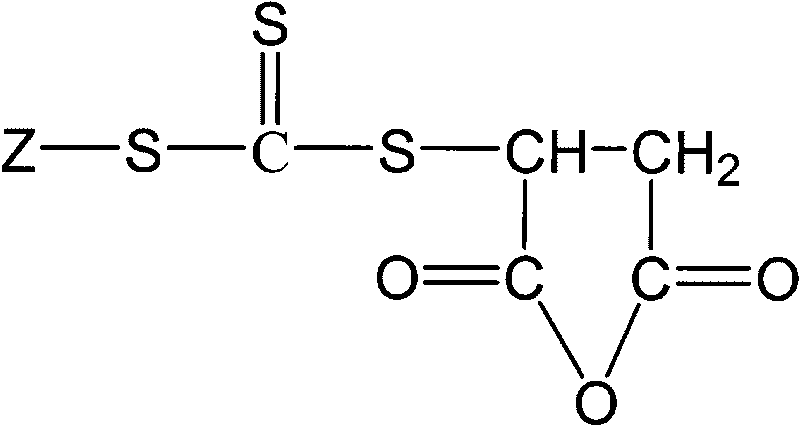
Trithiocarbonate chain transfer agent and preparation method thereof
A technology of trithiocarbonate and chain transfer agent, applied in the direction of organic chemistry, etc., can solve the problems of polymerization inhibition period, low chain transfer activity of dithioester, etc., achieve narrow molecular weight distribution, wide range of applicable monomers, The effect of high polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a dry 250 ml three-necked flask equipped with a stirrer, a 100°C thermometer and a 50 ml dropping funnel, 1.6 grams of sodium hydroxide and 53.5 grams of anhydrous ether were added. Stir at 100 rpm for 20 minutes under ice bath conditions. Slowly add 2.4 g of ethyl mercaptan dropwise, control the temperature at 10°C, and stir for 20 minutes. 3.05 grams of carbon disulfide was added dropwise, the solution began to turn yellow, and the solution was dropped in 10 minutes. Then, 10 grams of molecular sieves were added and stirred for 20 minutes. Add 3.9 grams of maleic anhydride, stir for 5 minutes, start to drop 2.4 grams of glacial acetic acid, and stop stirring after 0.5 hours of reaction to obtain a yellow solution with a small amount of white solid at the bottom. The solid was filtered off, and the solvent was evaporated to dryness with a rotary evaporator to obtain a yellow viscous liquid, which was the trithiocarbonate chain transfer agent S-ethyl-S'-(2-succinic ...
Embodiment 2
[0030] In a dry 500 ml three-necked flask equipped with a stirrer, a 100°C thermometer and a 50 ml dropping funnel, 3.2 grams of sodium hydroxide and 107 grams of anhydrous ether were added. Stir at 150 rpm for 30 minutes under ice bath conditions. 15.8 g of dodecyl mercaptan was slowly added dropwise, the solution gradually became cloudy, the temperature was controlled at 15° C., and a white slurry was obtained after the drop was completed, which was stirred for 30 minutes. 6.1 grams of carbon disulfide was added dropwise, and the solution began to turn yellow and thin. After 20 minutes, the solution was dropped to obtain a yellow suspension. There were a large amount of yellow flaky solids in the solution. Add 20 grams of molecular sieves and stir for 30 minutes. Add 7.8 grams of maleic anhydride, stir for 10 minutes, start to drop 4.8 grams of glacial acetic acid, and stop stirring after 1 hour of reaction to obtain a yellow solution with a small amount of white solid at th...
Embodiment 3
[0032] In a dry 500 ml three-necked flask equipped with a stirrer, a 100°C thermometer and a 50 ml dropping funnel, 3.2 grams of sodium hydroxide and 107 grams of anhydrous ether were added. Stir at 160 rpm for 35 minutes under ice bath conditions. Slowly add 22.3 grams of octadecyl mercaptan, the solution gradually becomes turbid, control the temperature at 15°C, and drop it in 35 minutes to obtain a white slurry, which is stirred for 35 minutes. 6.1 grams of carbon disulfide was added dropwise, and the solution began to turn yellow and thin. After 25 minutes, the solution was dripped to obtain a yellow suspension. There were a large amount of yellow flaky solids in the solution. 20 grams of molecular sieves were added and stirred for 35 minutes. Add 7.8 grams of maleic anhydride, stir for 15 minutes, start to drop 4.8 grams of glacial acetic acid, and stop stirring after 1 hour of reaction to obtain a yellow solution with a small amount of white solid at the bottom. Filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com