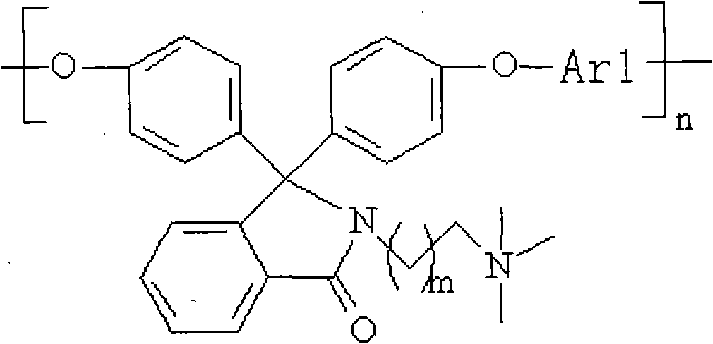
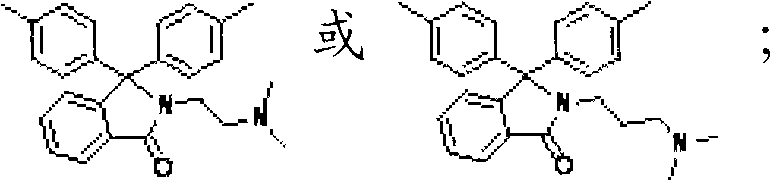
Quaternary ammonium lateral group-containing phenolphthalein type polyarylether, copolymer thereof and method for preparing same
A technology based on polyarylether and phenolphthalein, applied in the field of ion exchange membranes, can solve problems such as inability to eliminate batch differences, randomness in position and quantity, and impossibility of precise control, and achieve excellent solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The present invention also provides a preparation method for the phenolphthalein type polyarylether containing quaternary ammonium side groups, comprising the following steps:
[0051] a) phenolphthalein bisphenol monomers containing tertiary amine side groups and dihalogen monomers are dissolved in an aprotic polar solvent and reacted to obtain polyarylethers containing tertiary amine side groups,
[0052] The phenolphthalein bisphenol monomer containing tertiary amine side group is HO-Ar3-OH, and Ar3 is That is, N-dimethylaminoethyl-3,3'-bis(4-hydroxyphenyl)benzopyrrole (PPH-DMEDA) or N-dimethylaminopropyl-3,3'-bis(4-hydroxyphenyl) base) benzopyrrole (PPH-DMPDA);
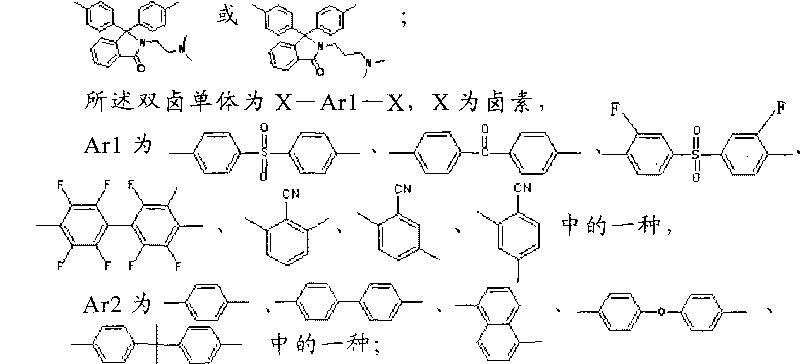
[0053] Dihalogen monomer is X-Ar1-X, X is halogen,
[0054] Ar1 is one of
[0055] In the reaction, the molar ratio of the phenolphthalein bisphenol monomer containing the tertiary amine side group to the dihalogen monomer is 1:1.
[0056] Reaction preferably uses catalyzer, and catalyzer is ...
Embodiment 1
[0078] Add 3.8846 grams of N-dimethylaminoethyl-3,3'-bis(4-hydroxyphenyl)benzopyrrolidone (PPH-DMEDA) in a three-necked flask equipped with mechanical stirring, condenser, and water separator (0.01mol), dichlorodiphenyl sulfone 2.8716 grams (0.01mol), potassium carbonate 2.1 grams (0.015mol), NMP (N-methylpyrrolidone) 35ml.
[0079] Stir at room temperature for 10 minutes under the protection of nitrogen, add 15 ml of toluene, raise the temperature to 150°C, evaporate the toluene after azeotropic removal of water for 4 hours, heat up to 180°C and polymerize for 10 hours to obtain a viscous solution, add DMF to dilute, filter out insoluble thing. Pour into water to precipitate a white solid, wash with water several times, and dry in vacuo. The intrinsic viscosity is 0.6dL / g.
[0080] Add 2 grams of the obtained white solid and 20 milliliters of NMP into a single-necked flask equipped with magnetic stirring and a condenser, and after fully stirring and dissolving, add 1 gram o...
Embodiment 2
[0084] Add 3.8846 grams of N-dimethylaminoethyl-3,3'-bis(4-hydroxyphenyl)benzopyrrolidone (PPH-DMEDA) in a three-necked flask equipped with mechanical stirring, condenser, and water separator (0.01mol), difluorodiphenyl sulfone 0.01mol, potassium carbonate 0.018mol, dimethyl sulfoxide 40ml.
[0085] Stir at room temperature for 10 minutes under the protection of nitrogen, add 15 ml of toluene, heat up to 140°C, evaporate the toluene after azeotropic water removal for 5 hours, heat up to 160°C and polymerize for 15 hours to obtain a viscous solution, add DMF to dilute, filter out insoluble thing. Pour into water to precipitate a white solid, wash with water several times, and dry in vacuo. The intrinsic viscosity is 0.9dL / g.
[0086] Add 2 grams of the obtained white solid and 20 milliliters of dimethyl sulfoxide into a single-necked flask equipped with magnetic stirring and a condenser, stir and dissolve, add 1 gram of methyl iodide, and react at 35 degrees for 9 hours. Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com