Method for preparing and separating stereotyped endoderm cells by using monolayer culture technology
A technology for finalizing endoderm and embryonic stem cells, which is applied in the biological field and can solve problems such as difficult identification of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
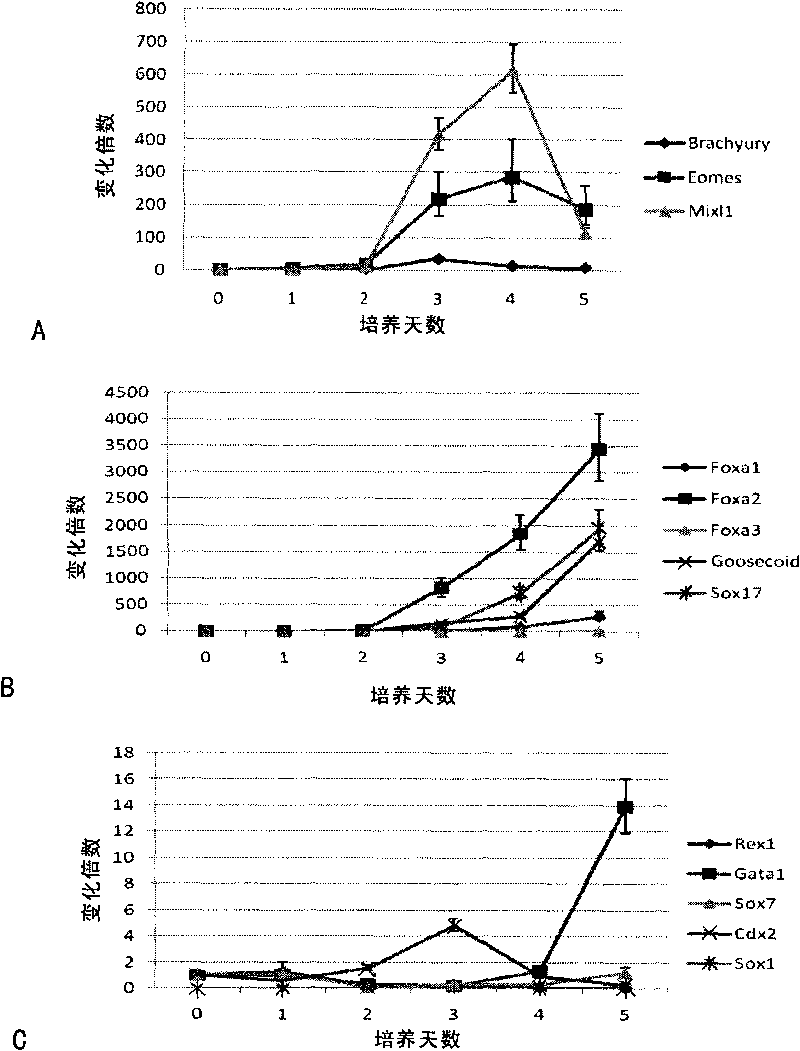
[0153] The present invention also includes the cell group containing definitive endoderm cells obtained by the aforementioned preparation method. In the cell group, the number of definitive endoderm cells accounts for more than 40% of the total number of cells, preferably more than 45% of the total number of cells; more preferably 50% or more of the total number of cells.
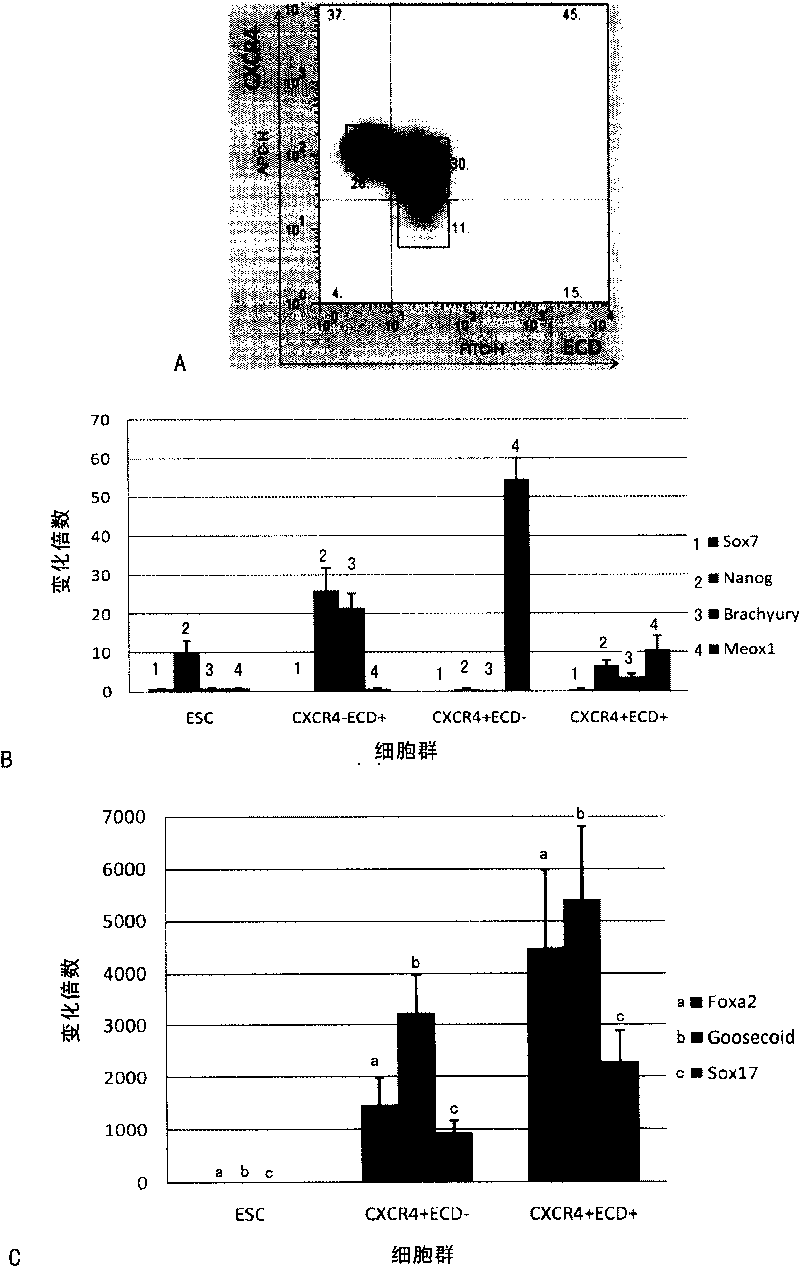
[0154] The present invention also includes the cell group with higher content of definitive endoderm cells separated by sorting technology. Preferably, cell groups positive for CXCR4 molecules and ECD molecules on the cell surface can be sorted, and in the cell group, definitive endoderm cells account for more than 80% of the total number of cells; preferably more than 90%.
[0155]As a preferred mode of the present invention, after the cell population containing definitive endoderm cells is obtained, the method further includes the step of: inducing the definitive endoderm cells to differentiate into a cel...
Embodiment 1
[0169] Example 1. Induction of differentiation of definitive endoderm cells
[0170] 1. Culture of Mouse Embryonic Stem Cells
[0172] Take an appropriate amount of ESGRO Complete Gelatin Solution (Millipore) into a culture bottle / dish, let it stand at room temperature for 30-60 minutes, suck off the gelatin solution, and set aside.
[0173] b) Passage of mouse embryonic stem cells
[0174] Every 2-3 days, mouse embryonic stem cells (purchased from ATCC, CRL-1821) were digested with ESGRO CompleteEnzyme-Free Dissociation Solution (Millipore) at 37°C for 5-10 minutes, and passaged at 1:3 to 1:5. Passage every 2-3 days. The composition of every 100ml culture fluid is as Table 5 formula 1 or formula 2.
[0175] When preparing the liquid culture medium, if the volume is insufficient, the balance is water.
[0176] table 5
[0177]
[0178] In the above table, 0.45M mono-dihydroxypropanethiol 0.033ml is equivalent to the final concentrati...
Embodiment 2
[0187] Example 2. Detection of surface markers CXCR4 and E-Cadherin (ECD) by flow cytometry
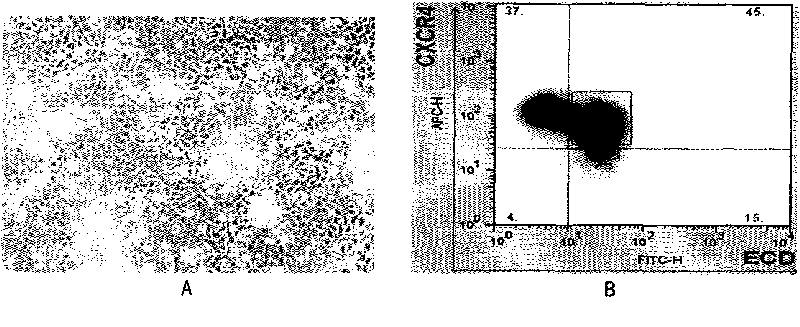
[0188] Suspend the digested cells in PBS containing 0.5% BSA, 5 Add 10 μl of antibody to each cell, add anti-mouse CXCR4-APC and anti-mouse ECD-PE antibodies (R&D) respectively, and incubate at 4°C for 30 minutes. Wash twice with PBS containing 0.5% BSA. Finally, 500 μl of PBS containing 0.5% BSA was used to suspend and detect on the FACS machine.
[0189] The inventors analyzed CXCR4 in differentiated cells by FACS + ECD + The proportion of the group, the analysis results see figure 1 b. Under normal circumstances, the induction efficiency of definitive endoderm cells is maintained at a level of 50%. The cells under culture conditions exhibit the typical epithelial-like morphology of definitive endoderm cells, and there are tight junctions between cells. The bright field cell morphology is shown in figure 1 a. figure 1 For each result, formula 1 medium in Table 6 was specific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com