Specific antibody against pesticide phosmet
An imophos-specific technology, applied in the field of immunochemistry of pesticide small molecule compounds and pesticide residue analysis, can solve the problems of excessive pesticide residues and affecting the health of agricultural product export trade, etc., achieving high similarity and good specificity and the effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
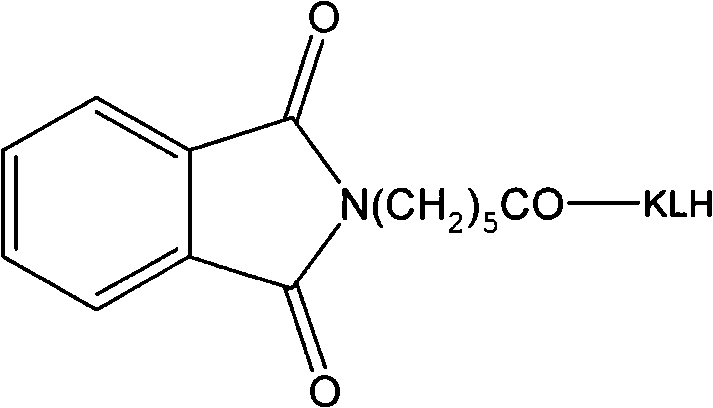
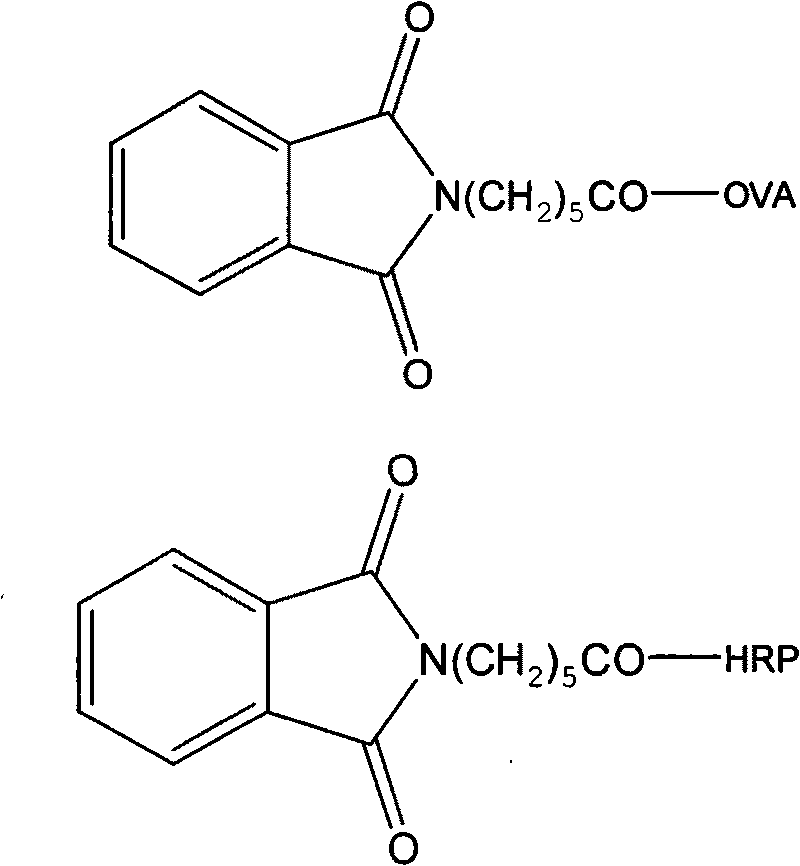
[0036] Hapten Synthesis and Antibody Preparation
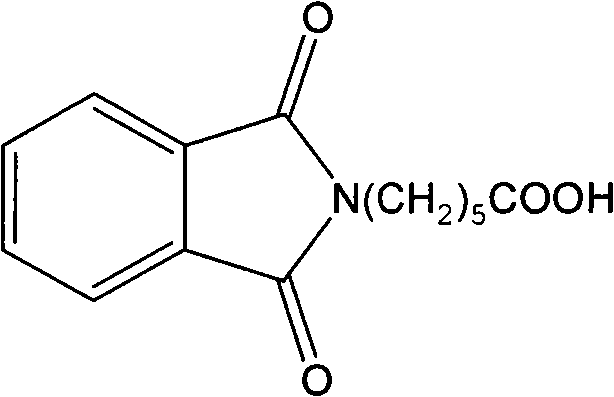
[0037] (1) Synthesis of N-phthalimido-6-aminocaproic acid:
[0038] Accurately weigh 0.1250g of aminocaproic acid, 0.0960g of anhydrous Na 2 CO 3 Dissolve in 5.0 mL of distilled water, add 0.255 g of N-phthalimide methyl ester, stir at room temperature until completely dissolved, and terminate the reaction in about 1 hour. Filtration, acid precipitation of the filtrate until the precipitation is complete, extraction with ethyl acetate, anhydrous Na 2 SO 4 After drying and evaporating the solvent, a white solid was obtained with a total yield of 90%. ;
[0039] (2) Synthesis of imophos hapten activated ester:
[0040] Weigh 0.5000g hapten, 0.2400g N-hydroxysuccinimide (NHS) is dissolved in 3ml anhydrous tetrahydrofuran, N 2Under protection, 2 ml of anhydrous tetrahydrofuran solution dissolved with 0.4500 g of DCC was added dropwise, and stirred overnight. The white precipitate was removed by filtration, the organic solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com