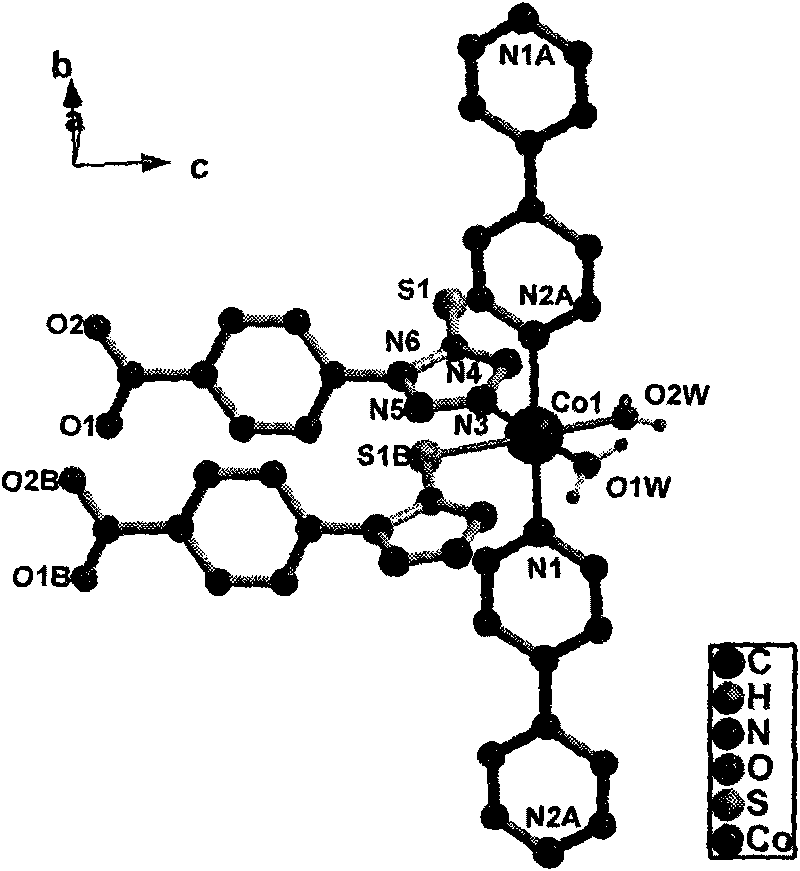
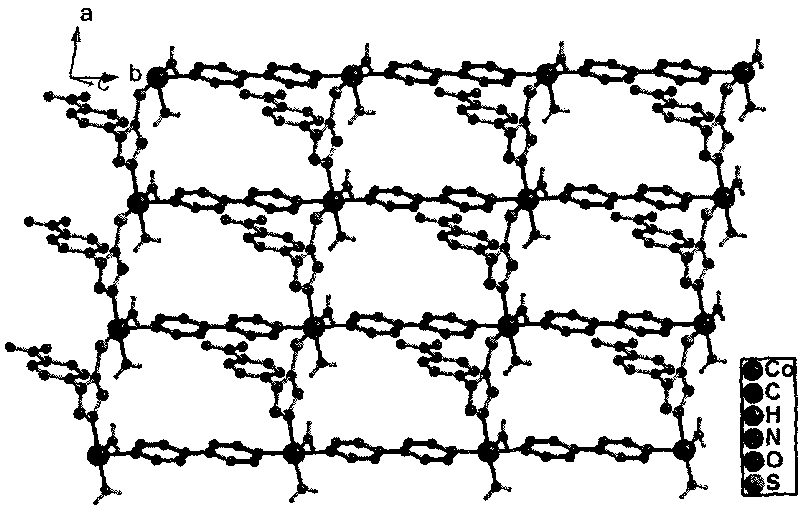
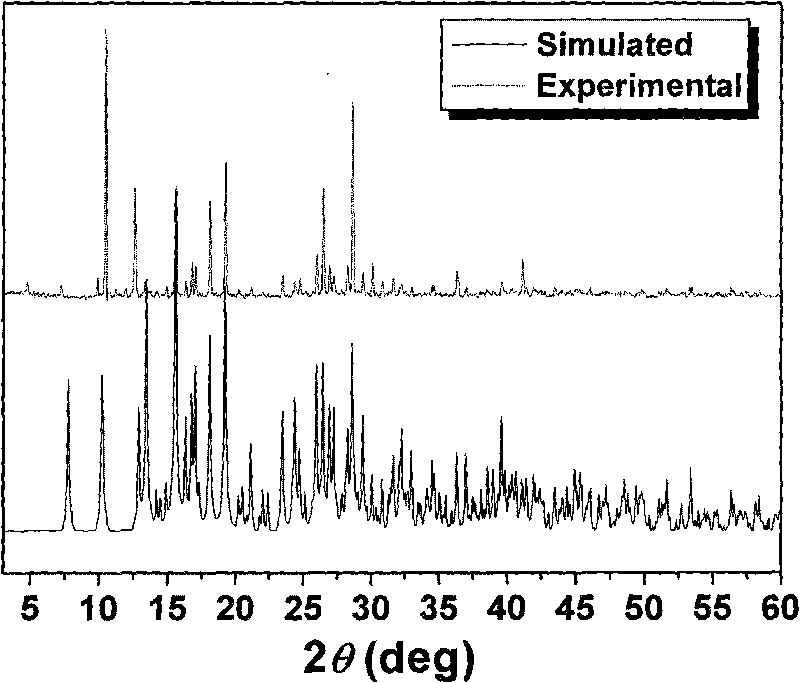
1-(4-carboxy phenyl)-5-sulfydryl-1H-tetrazole and 4, 4'-bipyridyl blending cobalt composition and preparation method thereof
A cobalt complex, carboxyphenyl technology, applied in the direction of cobalt organic compounds, organic materials/organic magnetic materials, etc., can solve the problems of poor repeatability, high risk, low yield, etc., and achieve mild and heavy conditions. The effect of good presentability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 0.1mmol Co(NO 3 ) 2 ·6H 2 O was placed at the bottom of the test tube, dissolved with 5mL of distilled water to form reaction solution A; 15ml of methanol and water mixed solution C with a volume ratio of 1:1 was added in the middle; 0.1mmol H 2 L and 0.1 mmol 4,4′-bipy were dissolved in 5 mL of methanol, and 0.05 mL of 2,6-lutidine was added to obtain reaction solution B, which was placed on the upper layer of the test tube. After being sealed and left standing for 24 days, a wine red massive single crystal appeared at the junction of the solution interface on the test tube wall. The crystals were washed successively with distilled water, methanol and ether, and dried. The yield was about 50%.
[0028] The main infrared absorption peaks are: 3447w, 3178w, 1605m, 1546m, 1413m, 1382m, 1363m, 1279m, 1218m, 1130m, 1103w, 1069w, 1040w, 1007m, 950w, 817m, 787m, 732w, 699w, 633m, 5629m, .
Embodiment 2
[0030] 0.1mmol Co(ClO 4 ) 2 ·6H 2 O was placed at the bottom of the test tube and dissolved with 4 mL of distilled water to form a reaction solution A; 16 ml of a mixed solution C of methanol and water at a volume ratio of 1:1.5 was added in the middle; 0.1 mmol H 2 L and 0.1 mmol 4,4′-bipy were dissolved in 4 mL of methanol, and 0.05 mL of 2,6-lutidine was added to obtain reaction solution B, which was placed on the upper layer of the test tube. After being sealed and left standing for 28 days, a wine red massive single crystal appeared at the junction of the solution interface on the test tube wall. The crystals were washed successively with distilled water, methanol and ether, and dried in vacuo. The yield was 55%.
[0031] The main infrared absorption peaks are: 3447w, 3178w, 1605m, 1546m, 1413m, 1382m, 1363m, 1279m, 1218m, 1130m, 1103w, 1069w, 1040w, 1007m, 950w, 817m, 787m, 732w, 699w, 633m, 5629m, .
Embodiment 3
[0033] 0.2mmol CoSO 4 ·7H 2 O was placed at the bottom of the test tube, dissolved with 5mL of distilled water to form reaction solution A; 12ml of mixed solution C of methanol and water with a volume ratio of 1:1.2 was added in the middle; 0.05mmol H 2 L and 0.05 mmol 4,4′-bipy were dissolved in 5 mL of methanol, and 0.03 mL of 2,6-lutidine was added to obtain reaction solution B, which was placed on the upper layer of the test tube. After sealing and standing for 30 days, a wine red massive single crystal appeared at the junction of the solution interface on the test tube wall. The crystals were washed successively with distilled water, methanol and ether, and dried in vacuo. The yield was 52%.
[0034] The main infrared absorption peaks are: 3447w, 3178w, 1605m, 1546m, 1413m, 1382m, 1363m, 1279m, 1218m, 1130m, 1103w, 1069w, 1040w, 1007m, 950w, 817m, 787m, 732w, 699w, 633m, 5629m, .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com