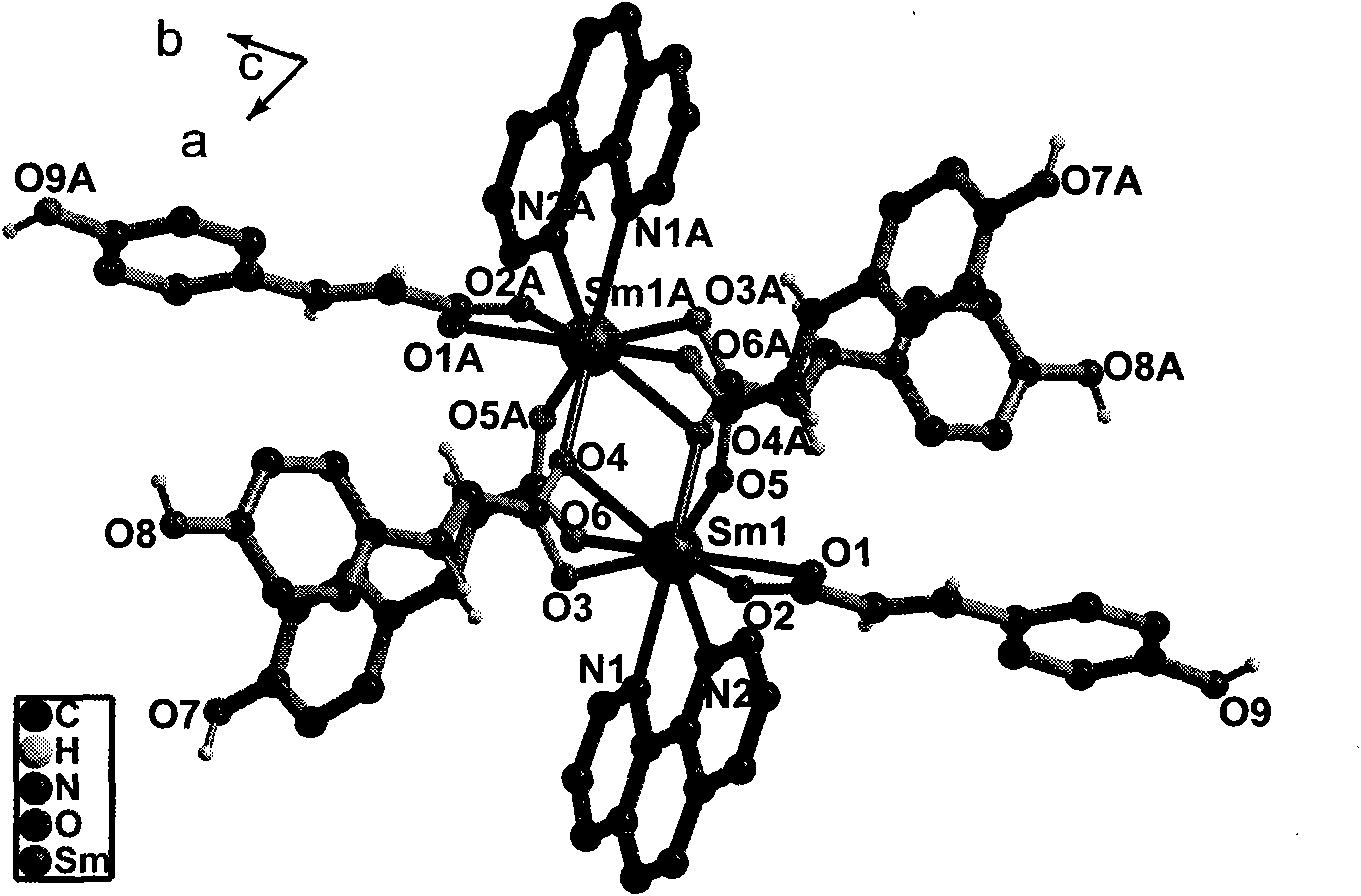
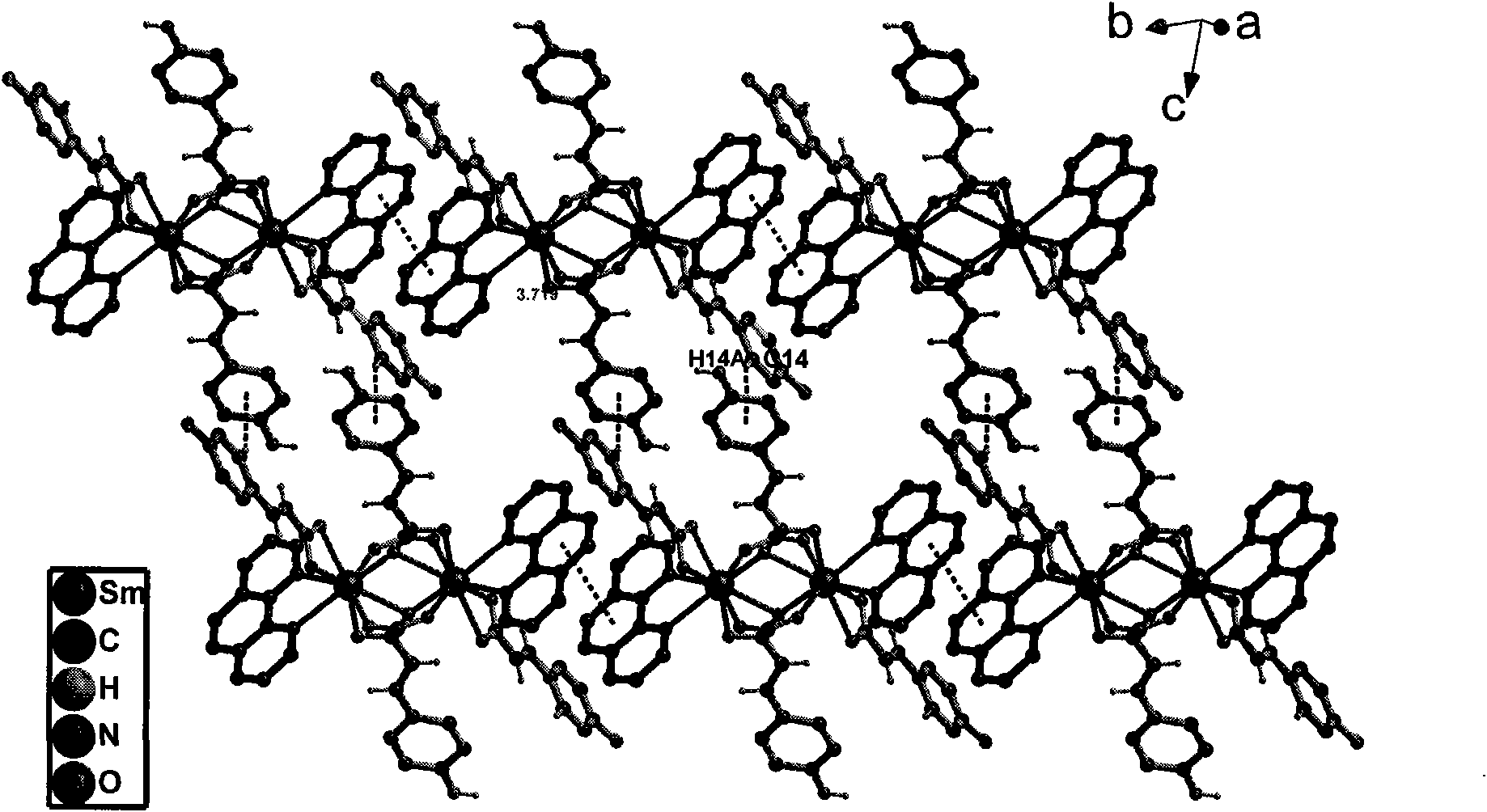
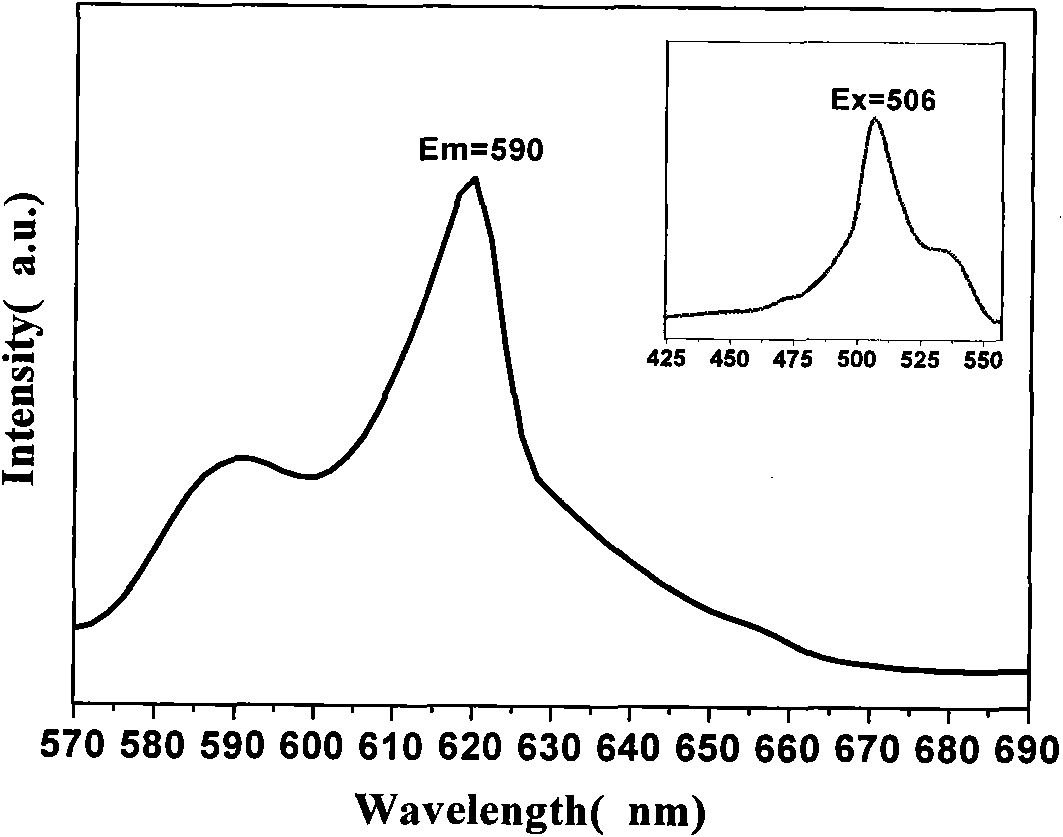
P-coumaric acid and o-phenanthroline mixed samarium complex, preparation method and application thereof
A technology of hydroxyphenylacrylic acid and carboxyl hydroxyphenylacrylic acid, which is applied in the field of rare earth metal complex materials, can solve the problems of difficult purification and separation of samples, high risk, high temperature, etc., and achieve high yield, overcome high temperature and high pressure, and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 0.1mmol Sm(NO 3 ) 3 ·6H 2 O is placed at the bottom of the test tube and dissolved with 5 mL of distilled water to form reaction solution A; 15 ml of a mixed solution C of methanol and water at a volume ratio of 1:1 is added in the middle; 0.05 mmol of HL and 0.05 mmol of phen are dissolved in 5 mL of methanol, and 2,6-dimethyl Base pyridine 0.017mL to obtain reaction solution B, placed in the upper layer of the test tube. After sealing and standing for 25 days, a yellow blocky single crystal appeared at the junction of the solution interface on the test tube wall. The crystals were washed successively with distilled water, ethanol and ether, and dried in vacuum, with a yield of about 50%.
[0026] The main infrared absorption peaks are: 3184s(br), 2816w, 1636s, 1607s, 1565s, 1512s, 1417vs, 1347w, 1287m, 1248s, 1168s, 1141w, 1101w, 1047w, 981m, 939w, 872w, 863m, 836s, 806w, 765w, 729m, 703w, 637w, 625w, 566w, 454w, 529m, 517w, 416w.
Embodiment 2
[0027] The preparation of embodiment 2 complexes
[0028] 0.2mmol Sm(ClO 4 ) 3 ·6H 2 O is placed at the bottom of the test tube and dissolved with 5 mL of distilled water; 20 mL of a mixed solution of methanol and water at a volume ratio of 1:1.2 is added in the middle; 0.08 mmol of HL and 0.08 mmol of phen are dissolved in 5 mL of methanol, and 0.023 mL of 2,6-lutidine is added The reaction solution B was obtained and placed on the upper layer of the test tube. After being sealed and left standing for 28 days, yellow massive single crystals appeared at the junction of the solution interface on the test tube wall. The crystals were washed successively with distilled water, ethanol and ether, and dried in vacuum. The yield was about 55%.
[0029] The main infrared absorption peaks are: 3184s(br), 2816w, 1636s, 1607s, 1565s, 1512s, 1417vs, 1347w, 1287m, 1248s, 1168s, 1141w, 1101w, 1047w, 981m, 939w, 872w, 863m, 836s, 806w, 765w, 729m, 703w, 637w, 625w, 566w, 454w, 529m, 517...
Embodiment 3
[0030] The preparation of embodiment 3 complexes
[0031] 0.24mmol Sm(SO 4 ) 3 ·8H 2 O is placed at the bottom of the test tube and dissolved with 4 mL of distilled water to form reaction solution A; 16 ml of a mixed solution C of methanol and water at a volume ratio of 1:1.1 is added in the middle; 0.10 mmol of HL and 0.10 mmol of phen are dissolved in 4 mL of methanol, and 2,6-dimethyl Base pyridine 0.02mL to obtain reaction solution B, placed in the upper layer of the test tube. After sealing and standing for 33 days, a yellow blocky single crystal appeared at the junction of the solution interface on the test tube wall. The crystals were washed successively with distilled water, ethanol and ether, and dried in vacuum, with a yield of about 57%.
[0032] The main infrared absorption peaks are: 3184s(br), 2816w, 1636s, 1607s, 1565s, 1512s, 1417vs, 1347w, 1287m, 1248s, 1168s, 1141w, 1101w, 1047w, 981m, 939w, 872w, 863m, 836s, 806w, 765w, 729m, 703w, 637w, 625w, 566w, 454...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com