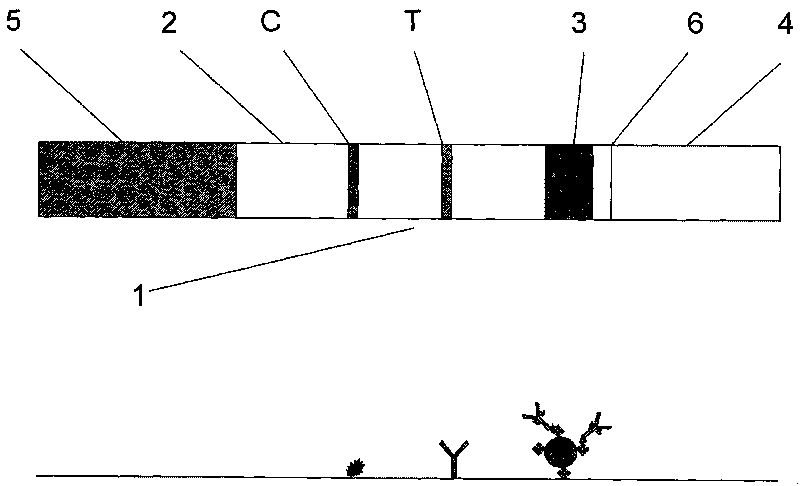
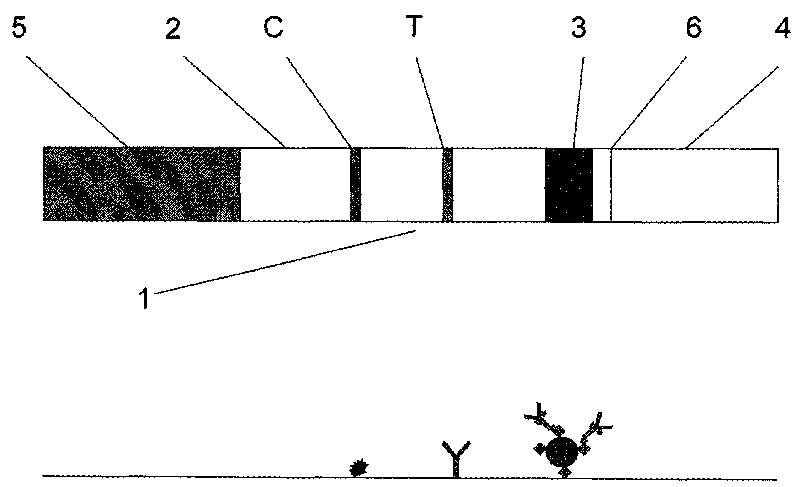
Magnetic immuno-chromatographic test paper strip for quantitatively detecting carcinoembryonic antigen in blood and preparation method thereof
A technology of magnetic immunochromatography and carcinoembryonic antigen, which is applied in the field of medical testing, can solve problems such as expensive instruments and equipment, hazards to operators, and difficulty in marketing, and achieve the effect of simplifying the preparation process and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Magnetic immunochromatographic test strip for quantitatively detecting carcinoembryonic antigen in blood and preparation method of test box
[0034] The preparation method of the test strip and the test carton of this embodiment includes the following steps:
[0035] A. Antibody preparation: select a commercial CEA paired antibody (Hytest, cat: 4CA30), and dialyze it overnight at 4°C with 20mM, pH7.2 (applicable to pH7.0-7.6) PBS.
[0036] B. Preparation of coating film:
[0037] Preparation of coating buffer: 0.02M pH 7.2 phosphate buffer (PB) is the coating buffer, 0.22μm microporous membrane filter sterilizes and store at 4°C for later use. The validity period is two weeks.
[0038] The preparation of the blocking solution: 0.02M phosphate buffered saline (PBS) containing 0.5% BSA, pH 7.2 (pH 7.0-7.6 are applicable), 0.22μm microporous membrane filter sterilized and stored at 4°C for later use , Valid for one week.
[0039] Preparation of coating film: use coating buffer (0.02...
Embodiment 2
[0068] Except for the steps in the preparation of streptavidinized magnetic particles: streptavidin makes the ratio of streptavidin to magnetic particles 1:3, the other steps are the same as in Example 1.
Embodiment 3
[0070] Except for the steps in the preparation of streptavidinized magnetic particles: streptavidin makes the ratio of streptavidin to magnetic particles 1:8, the other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com