Compound alpha-keto acid tablet and preparation method thereof
The technology of a ketoacid tablet and a compound formula is applied in the field of medicine, and can solve the problems of poor taste, poor stability, unpleasant smell and slight odor, and achieve the effects of simple preparation method, good stability and easy absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
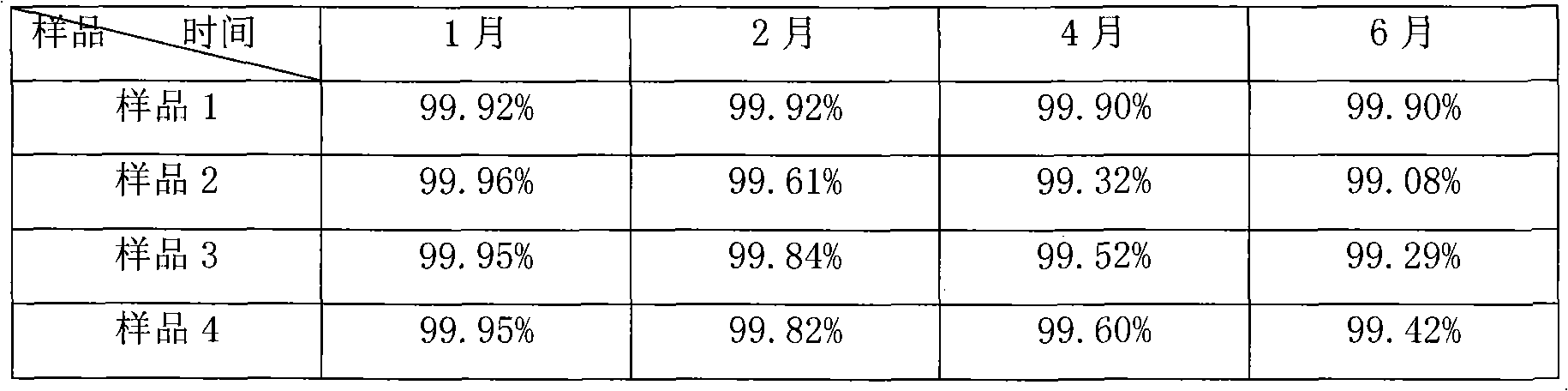
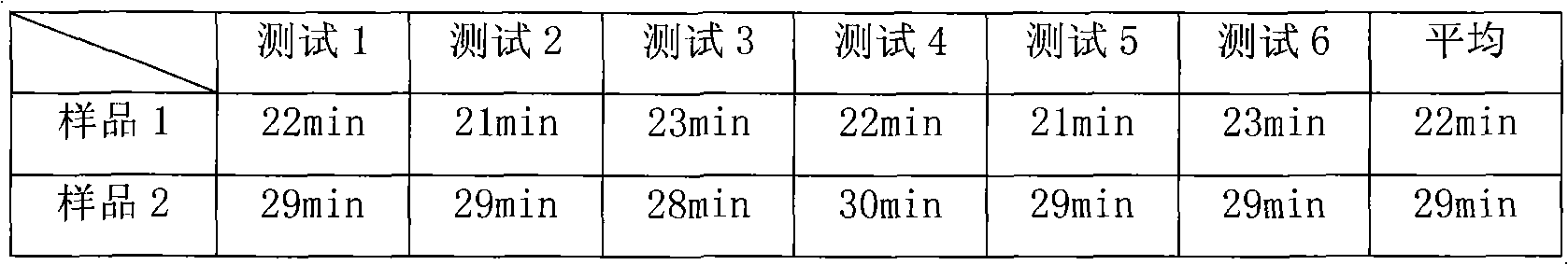
[0062] According to the following quality, prepare 1000 pieces of compound α-keto acid tablets
[0063] Calcium rac-Keto-Isoleucine 67g
[0064] Calcium Ketoleucine 101g
[0065] Calcium Ketophenylalanine 68g
[0066] Ketovaline Calcium 86g
[0067] Calcium R-Hydroxymethionine 59g
[0068] L-Lysine Acetate 105g
[0069] L-Threonine 53g
[0070] L-Tryptophan 23g
[0071] L-histidine 38g
[0072] L-Tyrosine 30g
[0073] Microcrystalline Cellulose 40g
[0074] Croscarmellose Sodium 50g
[0075] Povidone K30 10g
[0077] Micronized silica gel 5g
[0079] Purified water 150g
[0080] Preparation:
[0081] Each raw medicinal material is passed through a 100-mesh sieve respectively, and is set aside. Weigh povidone K30, slowly add it into an appropriate amount of water under stirring, and stir to dissolve.
[0082] Take by weighing the cross-linked sodium carboxymethyl cellulose of each principal agent that...
Embodiment 2
[0087] According to the following quality, prepare 1000 pieces of compound α-keto acid tablets
[0088] Calcium rac-Keto-Isoleucine 67g
[0089] Calcium Ketoleucine 101g
[0090] Calcium Ketophenylalanine 68g
[0091] Ketovaline Calcium 86g
[0092] Calcium R-Hydroxymethionine 59g
[0093] L-Lysine Acetate 105g
[0094] L-Threonine 53g
[0095] L-Tryptophan 23g
[0096] L-histidine 38g
[0097] L-Tyrosine 30g
[0098] Microcrystalline Cellulose 80g
[0099] Croscarmellose Sodium 200g
[0100] Povidone K30 50g
[0102] Micronized silica gel 20g
[0104] Purified water 400g
[0105] Preparation:
[0106] Each raw medicinal material is passed through a 100-mesh sieve respectively, and is set aside. Weigh povidone K30, slowly add it into an appropriate amount of water under stirring, and stir to dissolve.
[0107] Take by weighing each principal agent that has pretreated, adjuvant microcrystalline cel...
Embodiment 3
[0112] According to the following quality, prepare 1000 pieces of compound α-keto acid tablets
[0113] Calcium rac-Keto-Isoleucine 67g
[0114] Calcium Ketoleucine 101g
[0115] Calcium Ketophenylalanine 68g
[0116] Ketovaline Calcium 86g
[0117] Calcium R-Hydroxymethionine 59g
[0118] L-Lysine Acetate 105g
[0119] L-Threonine 53g
[0120] L-Tryptophan 23g
[0121] L-histidine 38g
[0122] L-Tyrosine 30g
[0123] Microcrystalline Cellulose 70g
[0124] Croscarmellose Sodium 120g
[0125] Povidone K30 30g
[0127] Micronized silica gel 20g
[0129] Purified water 250g
[0130] Preparation:
[0131] Each raw medicinal material is passed through a 100-mesh sieve respectively, and is set aside. Weigh povidone K30, slowly add it into an appropriate amount of water under stirring, and stir to dissolve.
[0132] Take by weighing the cross-linked sodium carboxymethyl cellulose of each principal agent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com