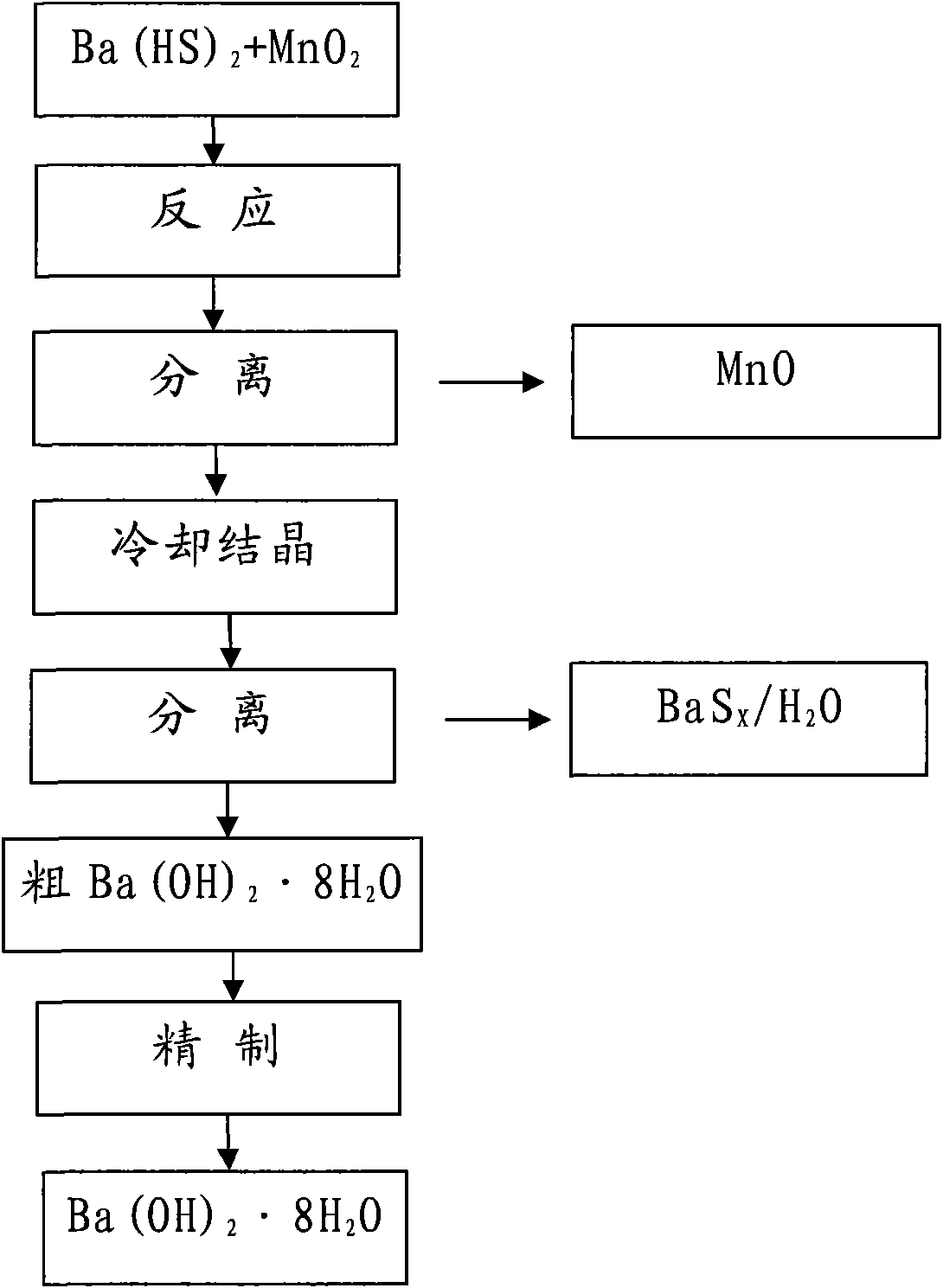
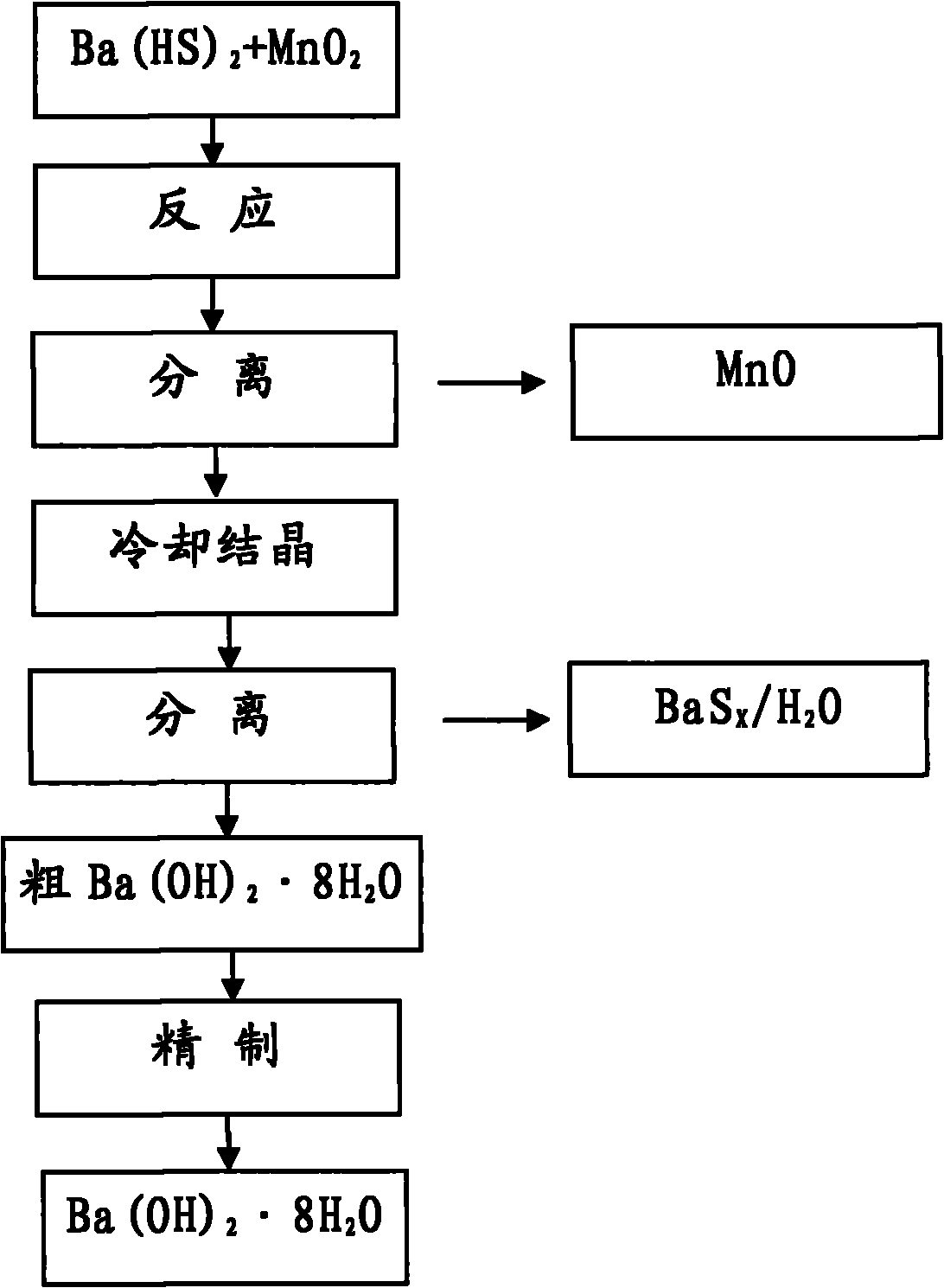
Method for preparing Ba(OH)2.8H2O
A 2.8H2O, solid-liquid separation technology, applied in the direction of calcium/strontium/barium oxide/hydroxide, etc., can solve the problems of high energy consumption and high cost, and achieve the effect of low energy consumption and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Will be taken from Guizhou Red Star Development Co., Ltd. [Ba 2+ ]0.83mol / L of Ba(HS) 2 Solution 4000ml is placed in the 5000ml beaker, adds 984g manganese oxide ore powder (MnO 2 38.7%, Fe8.6%), control the reaction temperature in the range of 70±5°C, close the stirring reaction for 1.5 hours, stop stirring, keep warm and settle for 30 minutes, absorb the supernatant with a latex tube, add 1000ml hot deionization to the original beaker After dilution with water, suction filter with insulation leak head, and prepare MnSO from the filter residue 4 , and the combined filtrates were sealed and cooled to 35°C.
[0014] The above solution is separated by suction filtration, and the filtrate reclaims barium carbonate and sulfur, and the crude Ba(OH) 2 ·8H 2 O press [Ba 2+ ] 2.0mol / L ingredients were heated and dissolved with deionized water, and 27.5% H was added at a ratio of 3.5ml / L 2 o 2 Sulfur removal. Heat to boil and maintain boiling for 15 minutes, then filter...
Embodiment 2
[0017] Will be taken from Guizhou Red Star Development Co., Ltd. [Ba 2+ ]0.70mol / L of Ba(HS) 2 Solution 4000ml is placed in the 5000ml beaker, adds 1232g manganese oxide ore powder (MnO 2 25.1%, Fe7.8%), control the reaction temperature within the range of 65±5°C, and react with airtight stirring for 2.0 hours, stop stirring, keep warm and settle for 1 hour, siphon the upper part of the clear night with a latex tube, add 1000ml hot deionized water, and use a heat preservation Separation by funnel suction filtration, preparation of MnSO from filter residue 4 , and the combined filtrates were sealed and cooled to 32°C.
[0018] The above solution was separated by suction filtration, and the filtrate recovered BaCO 3 With sulfur, crude Ba(OH) 2 ·8H 2 O press [Ba 2+ ]2.2mol / L was heated and dissolved in deionized water, and 27.5% H was added at a ratio of 3.0ml / L 2 o 2 Remove sulfur, heat to boil, maintain slight boiling for 15 minutes, then filter with slow qualitative f...
Embodiment 3
[0021] Will be taken from Guizhou Red Star Development Co., Ltd. [Ba 2+ ]1.0mol / L of Ba(HS) 2 Solution 4000ml is placed in the 5000ml beaker, adds 1232g manganese oxide ore powder (MnO 2 25.1%, Fe 7.8%), control the reaction temperature within the range of 85±5°C, and react with airtight stirring for 2.0 hours, stop stirring, keep warm and settle for 1 hour, use a latex tube to siphon the upper part to clear the night, add 1000ml hot deionized water, and use a heat preservation funnel Separation by suction filtration, preparation of MnSO from filter residue 4 , and the combined filtrates were sealed and cooled to 30°C.
[0022] The above solution was separated by suction filtration, and the filtrate recovered BaCO 3 With sulfur, crude Ba(OH) 2 ·8H 2 O press [Ba 2+ ]2.5mol / L was heated and dissolved in deionized water, and 27.5% H was added at a ratio of 3.0ml / L 2 o 2 Remove sulfur, heat to boil, maintain slight boiling for 15 minutes, then filter with slow qualitative...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com