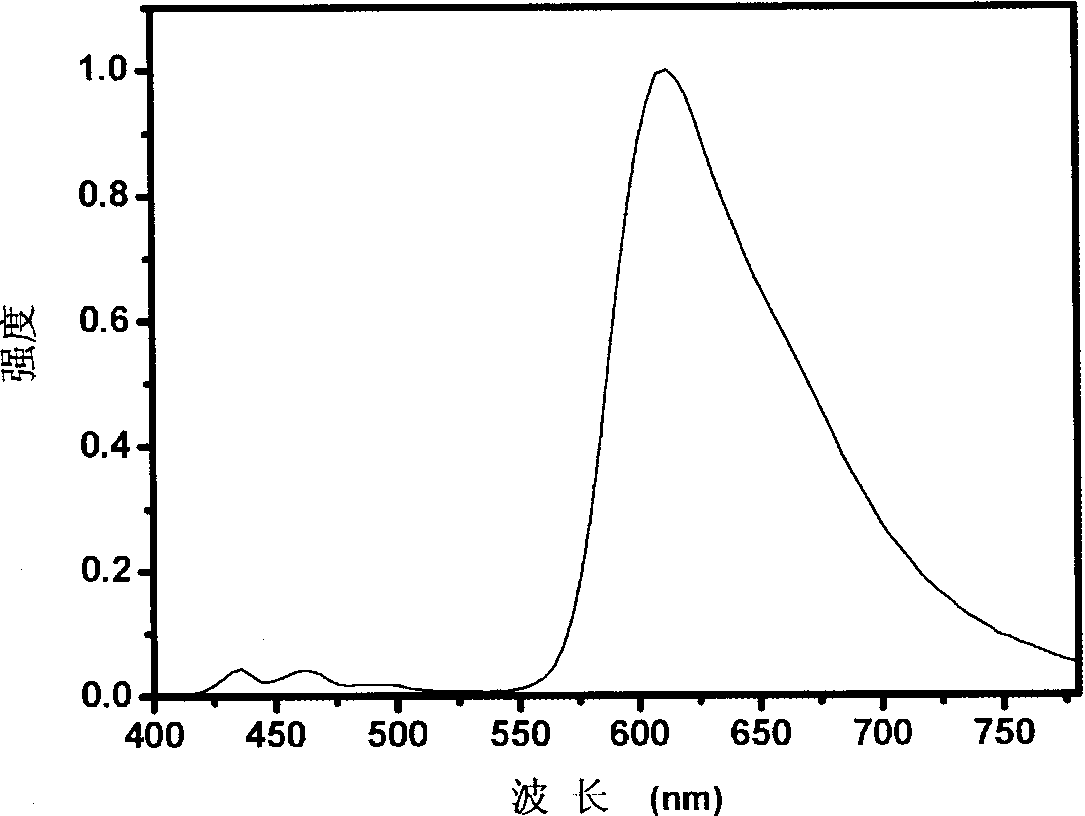
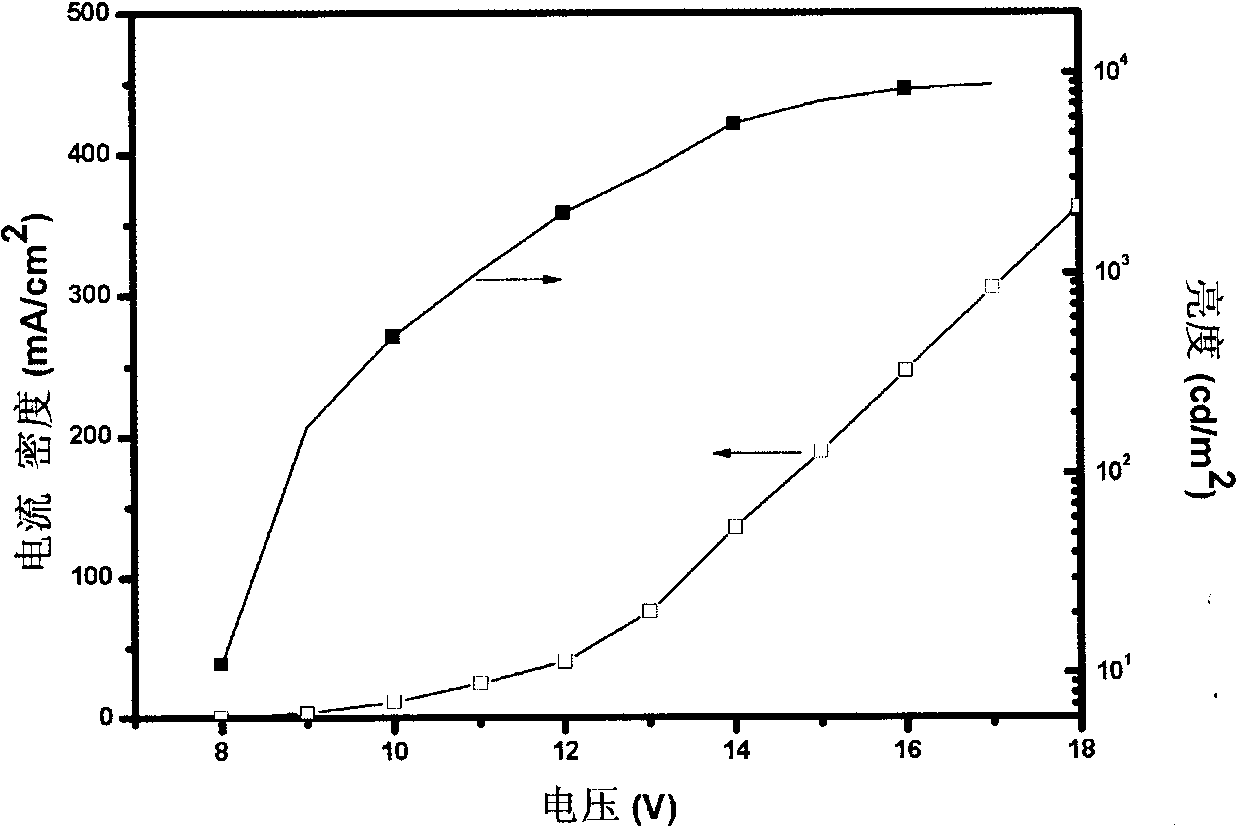
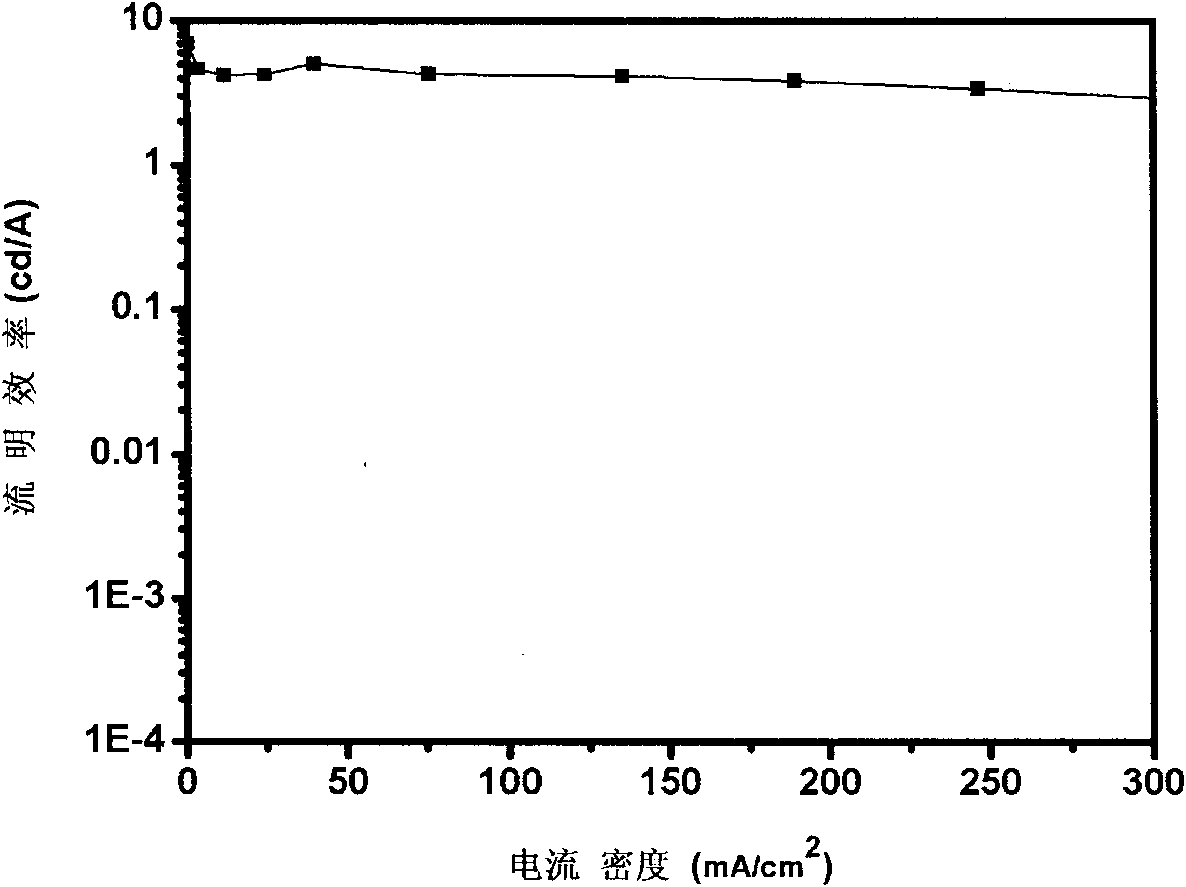
Red light conjugated polymer of side-chain quinoline ligand-containing iridium compound and light-emitting device
A technology of conjugated polymers and iridium complexes, which is applied in the manufacture of electrical solid devices, semiconductor devices, and semiconductor/solid-state devices. It can solve the problems of low luminous efficiency of light-emitting devices and achieve high luminous efficiency and stable performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Polymerization Example 1: Synthesis of phosphorescent polymer (PF0.5R0) with iridium complex content z=0.005, ie 0.5%:
[0073] The reaction formula is as follows:
[0074]
[0075] Under argon protection, 2,7-dibromo-9,9-dioctylfluorene (0.995mmol, 0.2715g) and DBrFR0 monomer (0.005mmol , 6.9mg), bis-boronate monomer of fluorene (0.5mmol, 0.2792g), 1% mmolPd (PPh 3 ) 4 , 20mg Aliguat336, 8mL toluene and 2mL 2M K 2 CO 3 Aqueous solution, under the protection of argon, stirred and reacted in the dark at 90°C for 24 hours, then added 100 mg of phenylboronic acid and 0.5 mL of bromobenzene successively, and reacted for 12 hours respectively. After the reaction, inject the reaction solution into methanol, filter and vacuum-dry the precipitated solid, extract with acetone for 48 hours, then dissolve methanol with dichloromethane for several times, and finally obtain a fibrous polymer after vacuum-drying.
[0076] The structural characterization of the obtained polyme...
Embodiment 4
[0088] Polymerization Example 4: Synthesis of phosphorescent polymer (PFCz0.5R0) with iridium complex content z=0.005, ie 0.5%:
[0089] The reaction formula is as follows:
[0090]
[0091] Under argon protection, 3,6-dibromo-N-decylcarbazole (0.495mmol, 0.2303g) and DBrCzR0 monomer (0.005mmol, 6.6 mg), bis-boronate monomer of fluorene (0.5mmol, 0.2792g), 1% mmolPd (PPh 3 ) 4 , 20mg Aliguat336, 8mL toluene and 2mL 2M K 2 CO 3 Aqueous solution, under the protection of argon, stirred and reacted in the dark at 90°C for 24 hours, then added 100 mg of phenylboronic acid and 0.5 mL of bromobenzene successively, and reacted for 12 hours respectively. After the reaction, inject the reaction solution into methanol, filter and vacuum-dry the precipitated solid, extract with acetone for 48 hours, then dissolve methanol with dichloromethane for several times, and finally obtain a fibrous polymer after vacuum-drying.
[0092] Structural characterization of the resulting polymer: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com