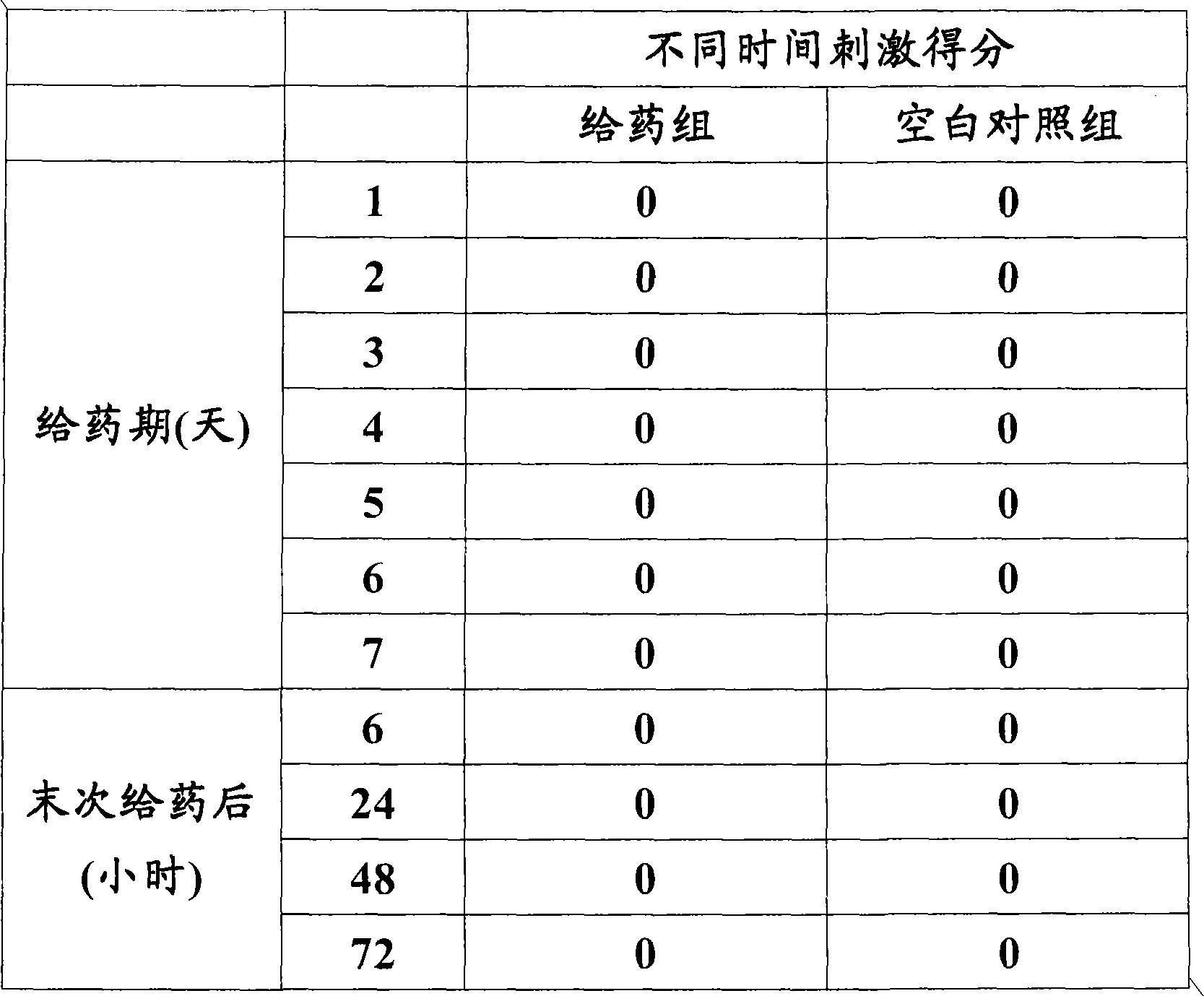
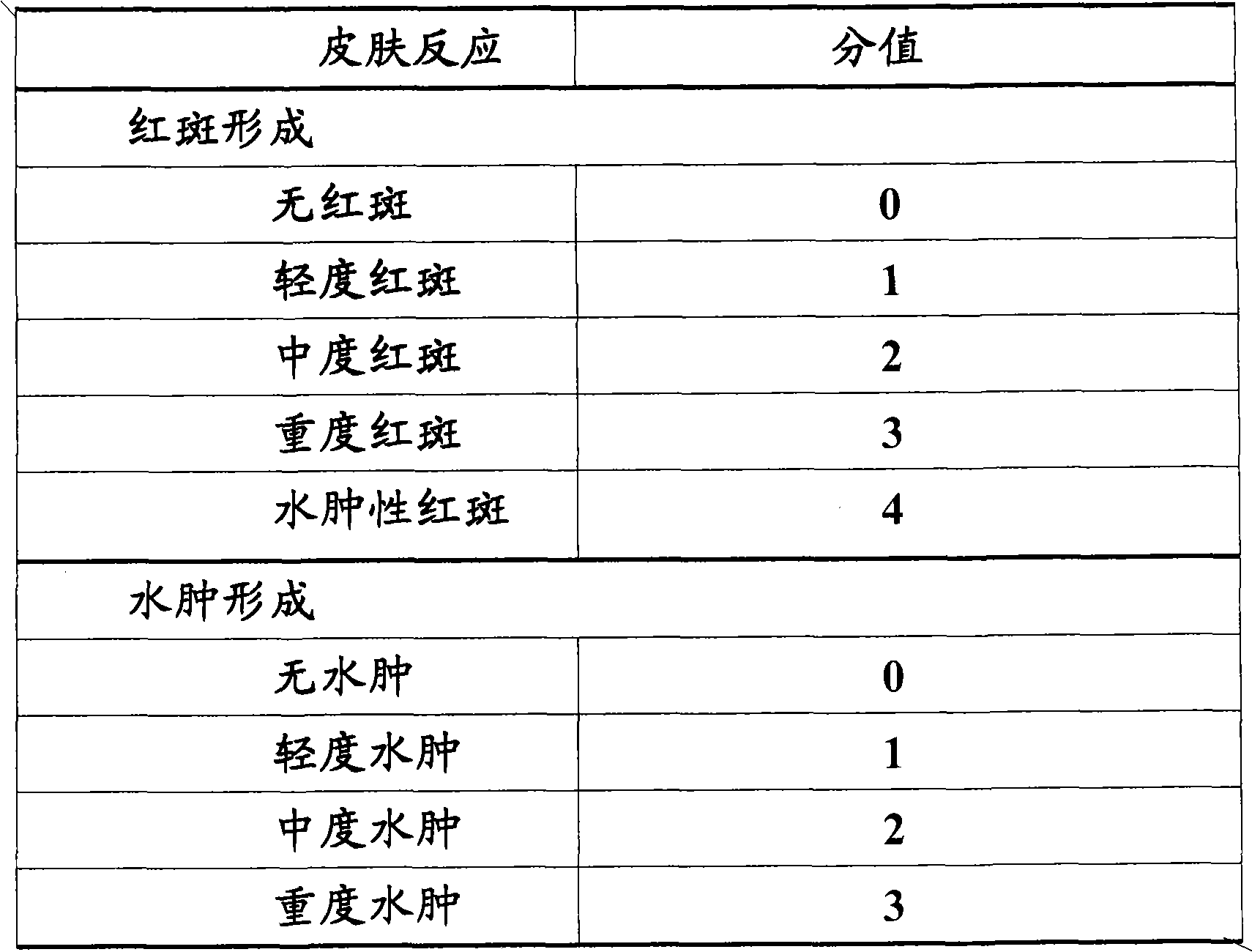
Eye drop of deproteinized calf blood extractive
A technology of eye drops and calf blood, which is applied in the direction of drug combination, drug delivery, active ingredients of hydroxyl compounds, etc., can solve the problems of unsatisfactory stability and unsuitability for ophthalmic preparations, and achieve easy control of the production process and clear appearance , low irritation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] prescription:
[0028] Calf blood deproteinized extract 200ml
[0029] Phenoxyethanol 5.0g
[0030] Benzalkonium Chloride 0.1g
[0031] Oligochitosan 0.5g
[0032] Appropriate amount of water for injection
[0033]
[0034] Full volume 1000ml
[0035] Preparation Process:
[0036] Weigh the prescribed amount of phenoxyethanol, benzalkonium chloride, and chitosan oligosaccharide under sterile conditions, heat and dissolve with an appropriate amount of water for injection, cool to room temperature, and add calf blood deproteinized extract and water for injection to the full amount. First filter with a 0.45 μm microporous membrane, then filter and sterilize with a 0.22 μm microporous membrane, and fill to obtain eye drops. The molecular weight of chitosan oligosaccharide used in this embodiment is below 5000.
Embodiment 2
[0038] prescription:
[0039] Calf blood deproteinized extract 200ml
[0040] Phenoxyethanol 5.0g
[0041] Ethylparaben 0.1g
[0042] Oligochitosan 0.5g
[0043] Appropriate amount of water for injection
[0044]
[0045] Full volume 1000ml
[0046] Preparation Process:
[0047] Weigh the prescribed amount of phenoxyethanol, ethylparaben, and chitosan oligosaccharide under sterile conditions, heat and dissolve with an appropriate amount of water for injection, cool to room temperature, and add calf blood deproteinized extract and water for injection to the full amount. First filter with a 0.45 μm microporous membrane, then filter and sterilize with a 0.22 μm microporous membrane, and fill to obtain eye drops. The molecular weight of chitosan oligosaccharide used in this embodiment is below 3000.
Embodiment 3
[0049] prescription:
[0050] Calf blood deproteinized extract 200ml
[0051] Chlorobutanol 1.0g
[0052] Benzalkonium Chloride 0.1g
[0053] Oligochitosan 1.0g
[0054] Appropriate amount of water for injection
[0055]
[0056] Full volume 1000ml
[0057] Preparation Process:
[0058] Weigh the prescribed amount of chlorobutanol, benzalkonium chloride, and chitosan oligosaccharide under sterile conditions, heat and dissolve with an appropriate amount of water for injection, cool to room temperature, and add calf blood deproteinized extract and water for injection to the full amount. First filter with a 0.45 μm microporous membrane, then filter and sterilize with a 0.22 μm microporous membrane, and fill to obtain eye drops. The molecular weight of chitosan oligosaccharide used in this embodiment is below 1500.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com