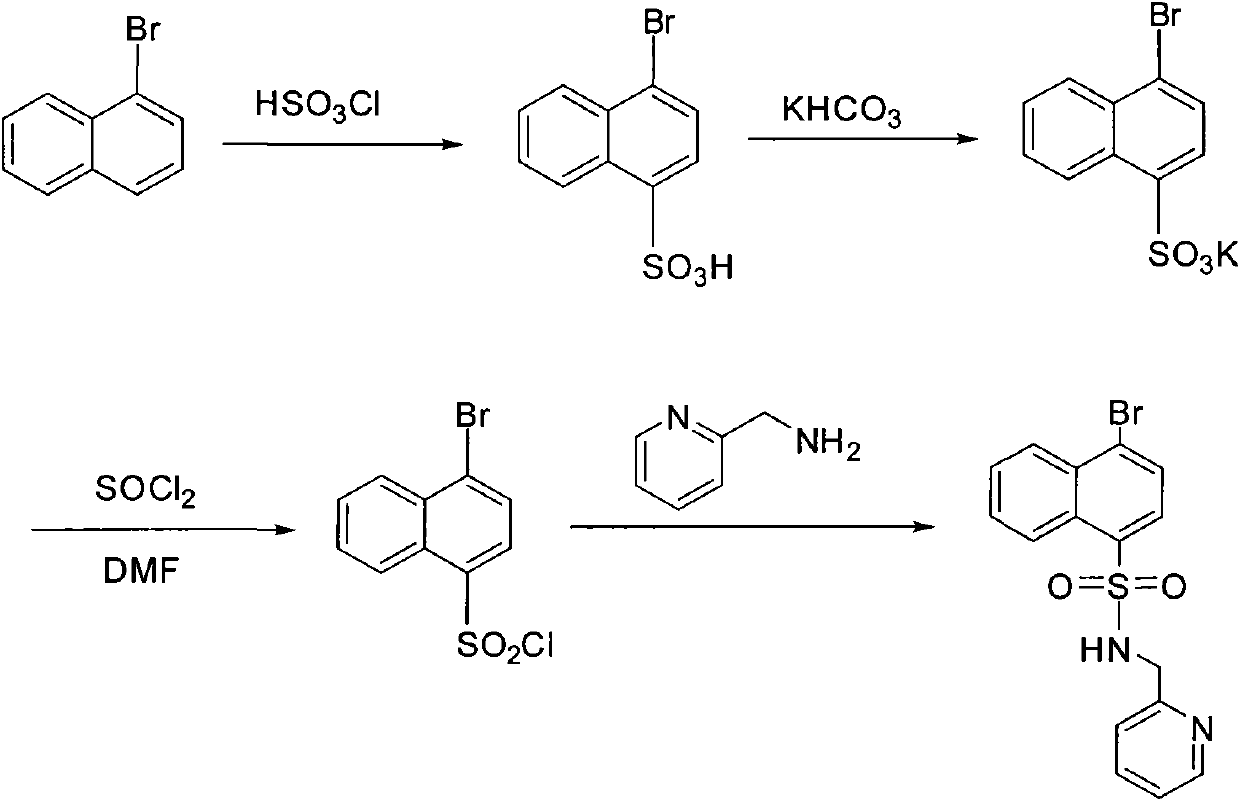
Preparation method of 4-bromo-N- (pyridine-2-methyl) naphthalene-1-sulfonamide compound
The technology of a compound, bromonaphthalene, is applied in the field of preparation of the compound 4-bromo-N-naphthalene-1-sulfonamide, achieving the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 1,4-bromo-N-(pyridine-2-methyl)naphthalene-1-sulfonamide
[0022] 1) Synthesis of potassium 4-bromonaphthalene-1-sulfonate
[0023] Add 41.4g (0.2mol) of 1-bromonaphthalene to a 250mL three-neck round bottom flask, 150mL CCl 4 As a solvent, slowly add 23.3g (0.2mol) of chlorosulfonic acid dropwise in an ice-salt bath at a temperature of 0-5°C. After the drop is complete, stir at room temperature for 12 hours. A large amount of gray solid precipitates. Pour the reaction solution into 200mL of ice water and the solid dissolves. ,Liquid separation. KHCO for aqueous phase 3 Adjust the pH to 7, a large amount of white solids precipitated, filtered and dried to obtain 52.7 g of potassium 4-bromonaphthalene-1-sulfonate with a yield of 81%.
[0024] 2) Synthesis of 4-bromonaphthalene-1-sulfonyl chloride
[0025] Add 32.5 g (0.1 mol) of 4-bromonaphthalene-1-sulfonic acid potassium and 50 mL SOCl to the 100 mL round bottom flask prepared in step 1)...
Embodiment 2
[0033] The synthesis of embodiment 2,4-bromo-N-(pyridine-2-methyl)naphthalene-1-sulfonamide
[0034] The preparation method is basically the same as in Example 1, the difference being that in step 3), a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:3 is used as an eluent for column chromatography to obtain 4-bromo-N-(pyridine -2-Methyl)naphthalene-1-sulfonamide 11.31 g, yield 60%.
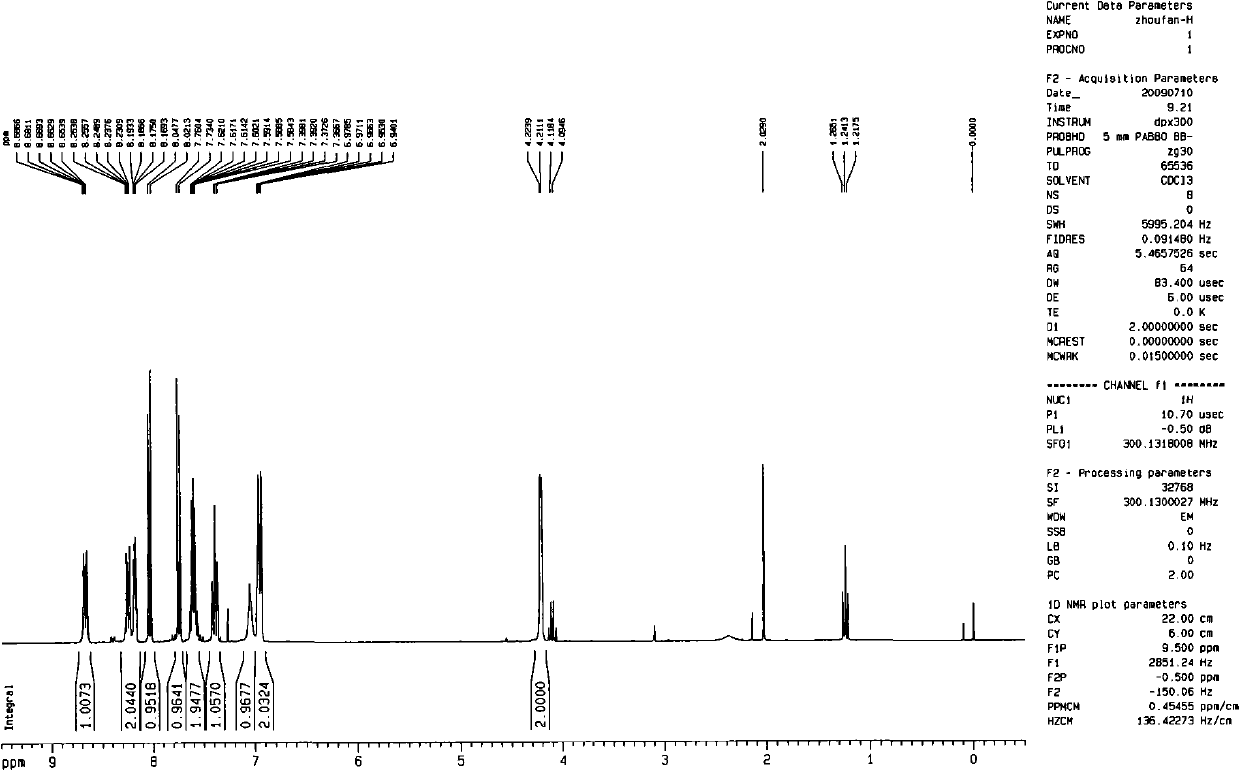
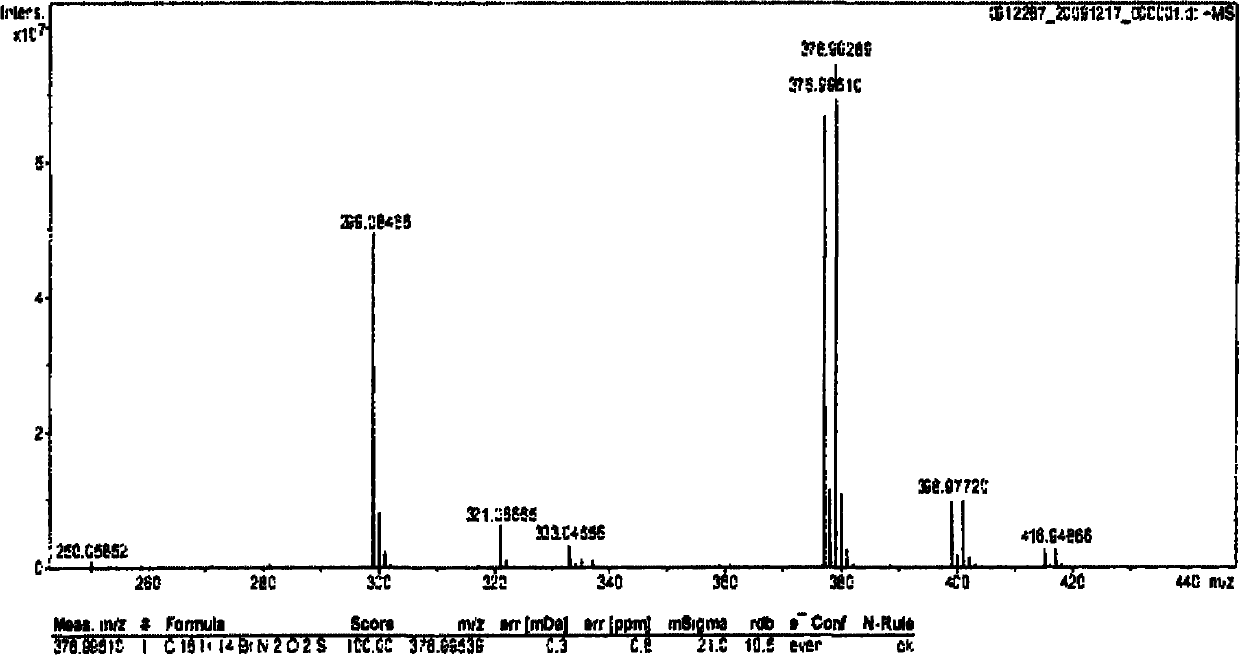
[0035] According to hydrogen spectrum and mass spectrum, it is proved that the compound synthesized in this example is consistent with the Pyrabactin structural formula reported in the literature.
Embodiment 3
[0036] The synthesis of embodiment 3,4-bromo-N-(pyridine-2-methyl)naphthalene-1-sulfonamide
[0037] The preparation method is basically the same as in Example 1, the difference being that in step 3), a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:5 is used as an eluent for column chromatography to obtain 4-bromo-N-(pyridine -2-methyl)naphthalene-1-sulfonamide 11.69 g, yield 62%.
[0038] According to hydrogen spectrum and mass spectrum, it is proved that the compound synthesized in this example is consistent with the Pyrabactin structural formula reported in the literature.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com