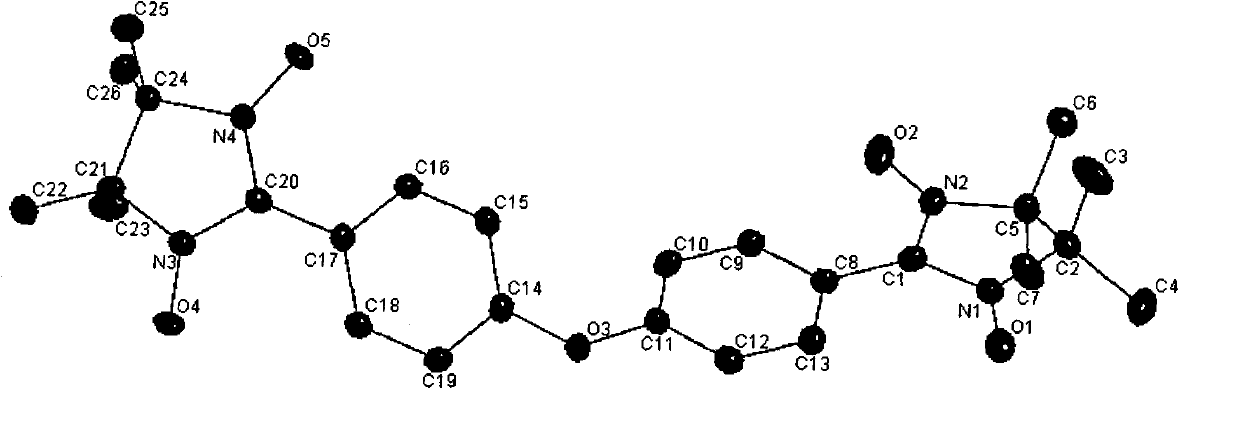
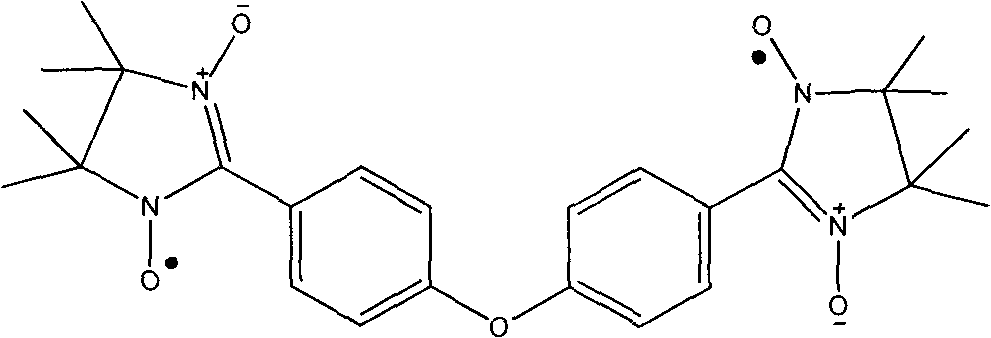
4,4-di(4,4,5,5-tetramethyl imidazoline-3-oxidation-1-oxy radical) phenyl ether and preparation method thereof
A tetramethylimidazoline, free radical technology, applied in 4 fields, can solve the problems of low yield, difficult, difficult to synthesize triradicals, etc., and achieves the effects of high yield, few synthesis steps, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0015] Synthesis of 2,3-Dimethyl-2,3-Dihydroxyaminobutane
[0016] Mix 90 grams (1.0mol) of 2-nitropropane and 168mL of 6N sodium hydroxide solution, cool in an ice-water bath, stir electromagnetically for half an hour, slowly add 80g (0.5mol) of liquid bromine dropwise, after the dropwise addition, add 300mL Continue to stir with absolute ethanol for half an hour, then reflux at 85°C for 3 hours, white flaky crystals are precipitated, naturally cool to room temperature, filter with suction, wash twice with dilute sodium hydroxide solution, wash several times with distilled water, and dry , to obtain 2,3-dimethyl-2,3-dinitrobutane in the form of 60 g of white flaky crystals, with a yield of 66% and a melting point of 129-130°C.
[0017] Dissolve 10 grams of ammonium chloride in 180 mL of a 1:1 mixed solvent of ethanol: water, add 17.5 grams of 2,3-dimethyl-2,3-dinitrobutane in an ice-water bath, and stir for half hour, slowly add purified zinc powder 40 grams in 3 hours with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com