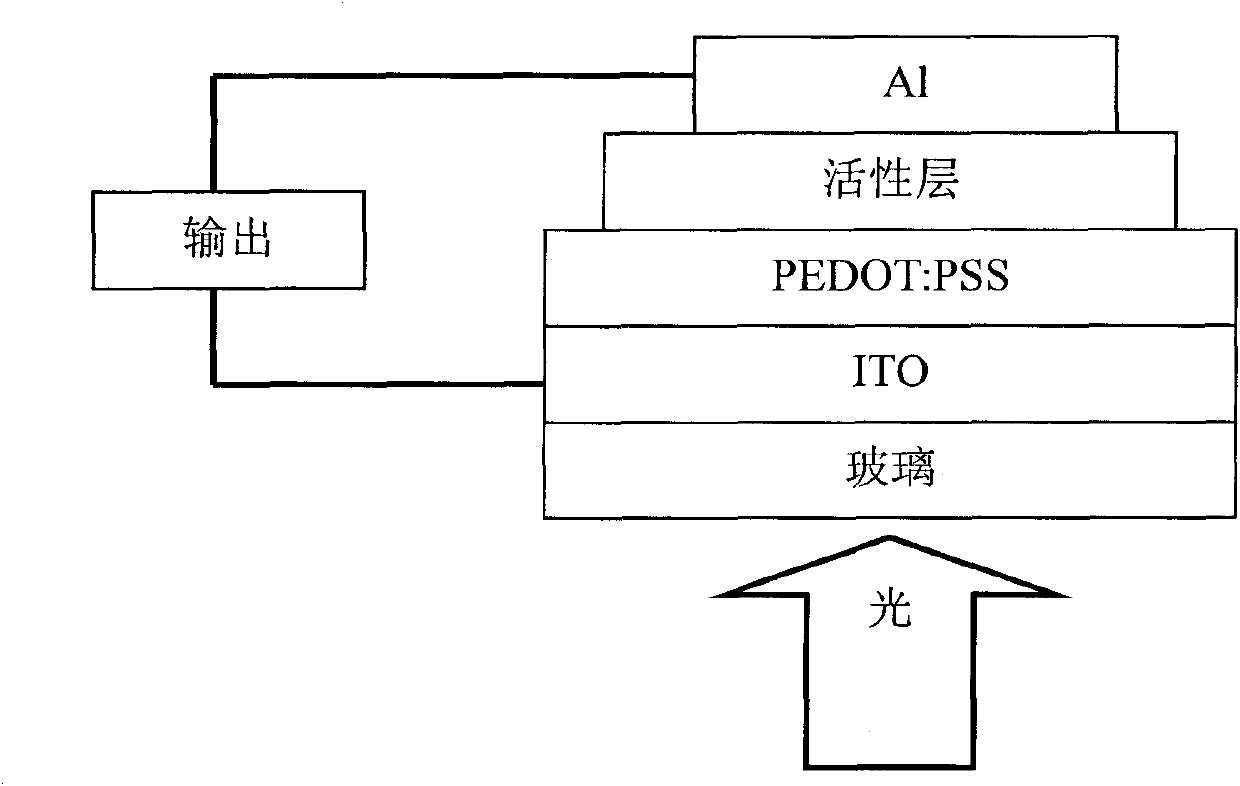
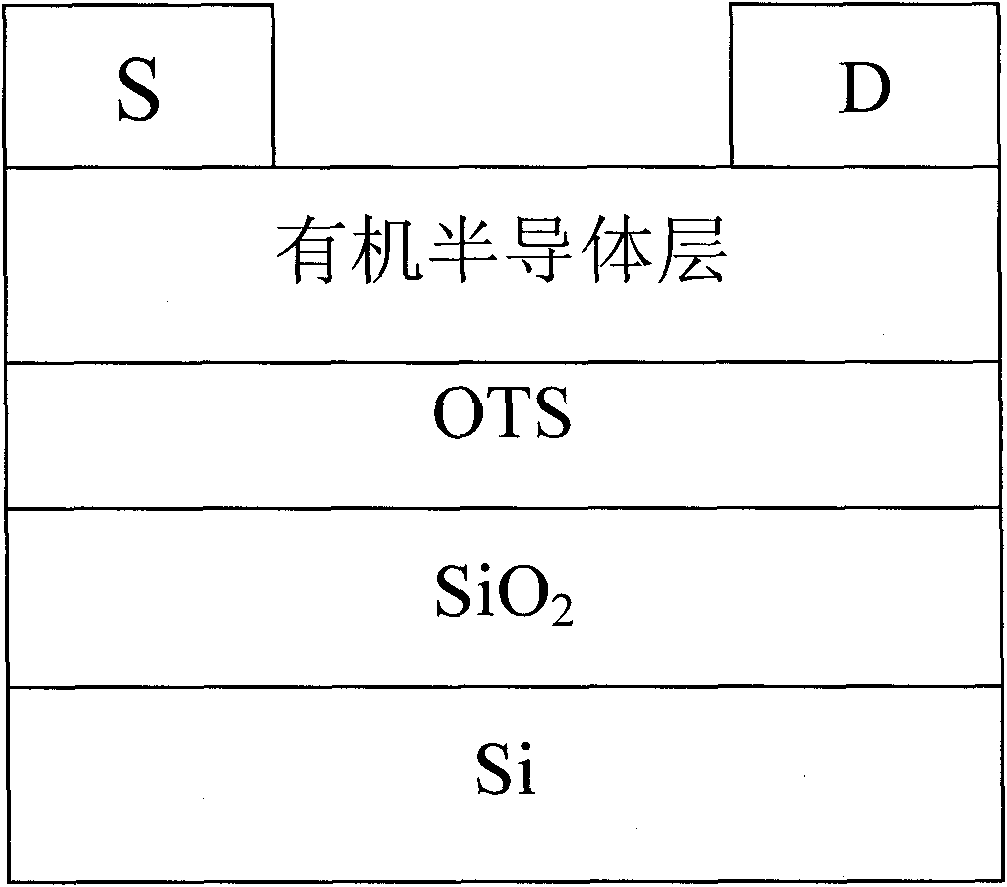
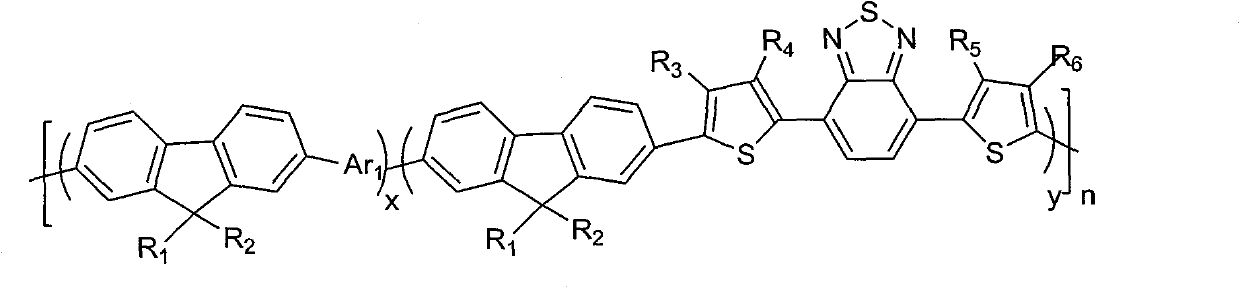
Fluorene copolymer and preparation method, application and polymer solar cell device thereof
A technology of copolymers and fluorenes, which is applied in the field of polymer materials, can solve the problems of narrow spectral response range, unsatisfactory solar emission spectral matching, and restrict the development of such materials, and achieves improved solubility and excellent reduction reversibility. , the effect of good thermal stability and environmental stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1. This embodiment discloses a fluorene copolymer with the following structure: (x=0.5, y=0.5, n=100)
[0051]
[0052] The preparation steps of above-mentioned fluorene copolymer are as follows:
[0053] 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluorene:
[0054]
[0055] Add 1.44mL (2.93M) of n-butyllithium solution to a reaction flask containing 1.10g of 2,7-dibromo-9,9-dioctylfluorene and 20mL of tetrahydrofuran at -78°C under nitrogen. After stirring for 2 hours, slowly add 1.00 mL of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane dropwise, return to room temperature, and continue stirring for 24 hours . After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, washed with petroleum ether, and precipitated with methanol to obtain a solid product.
[0056] MALDI-TOF-MS (m / z): 642.5 (M + ).
[0057] Two,...
Embodiment 2
[0069] Embodiment 2. This embodiment discloses a fluorene copolymer with the following structure: (x=0.1, y=0.9, n=10)
[0070]
[0071] The preparation steps of above-mentioned fluorene copolymer are as follows:
[0072] 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylfluorene:
[0073]
[0074] Add 11.00mL (2.00M) of n-butyllithium solution to a reaction flask containing 3.52g of 2,7-dibromo-9,9-dimethylfluorene and 50mL of tetrahydrofuran at -78°C under nitrogen. After stirring for 2 hours, slowly add 5.00 mL of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane dropwise, return to room temperature, and continue stirring for 28 hours . After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, washed with petroleum ether, and precipitated with methanol to obtain white needle crystals.
[0075] MALDI-TOF-MS (m / z): 446.2 (M + ).
[00...
Embodiment 3
[0090] Embodiment 3. This embodiment discloses a fluorene copolymer with the following structure: (x=0.9, y=0.1, n=26)
[0091]
[0092] The preparation steps of above-mentioned fluorene copolymer are as follows:
[0093] 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-bieicosylfluorene:
[0094]
[0095]Add 15.40mL (2.00M) of n-butyllithium solution to a reaction flask containing 8.85g of 2,7-dibromo-9,9-dieicosylfluorene and 110mL of tetrahydrofuran at -78°C under nitrogen After stirring for 3 hours, slowly add 5.20mL 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane dropwise, return to room temperature, and continue stirring 44 hours. After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, washed with petroleum ether, and precipitated with methanol to obtain a solid product.
[0096] MALDI-TOF-MS (m / z): 979.2 (M + ).
[0097] 2. Prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com