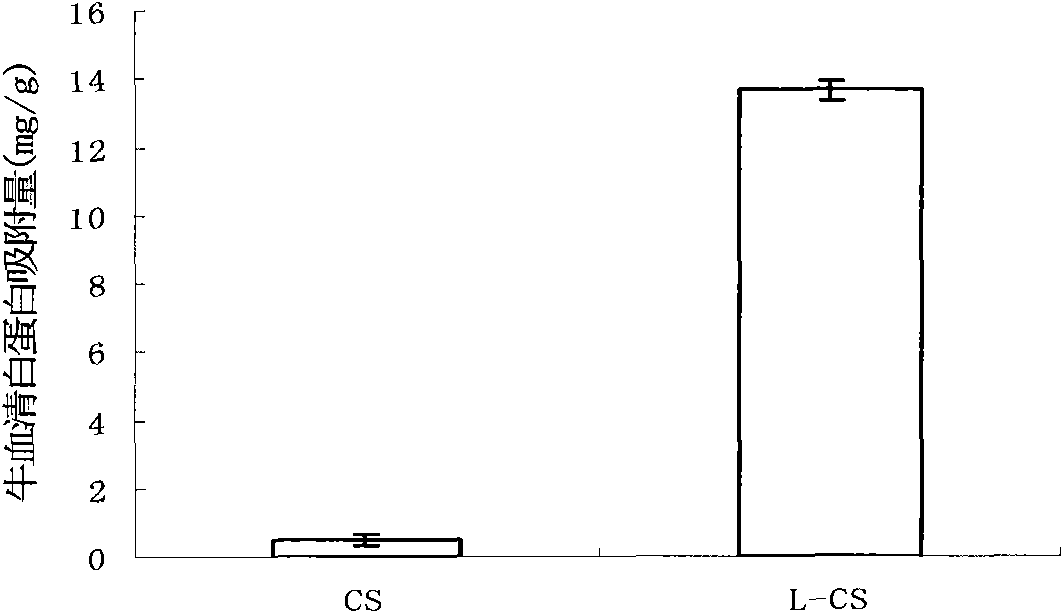
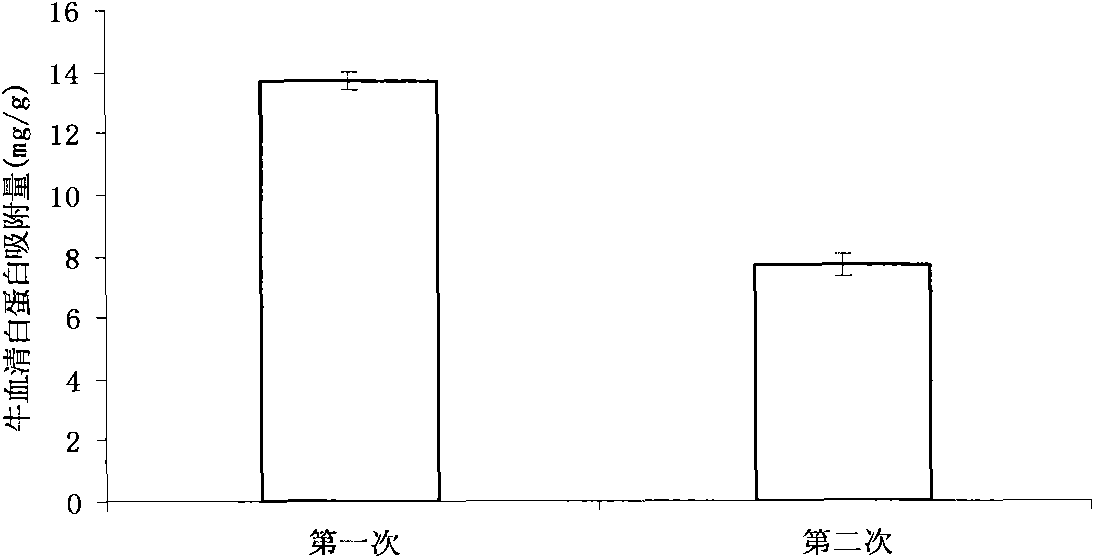
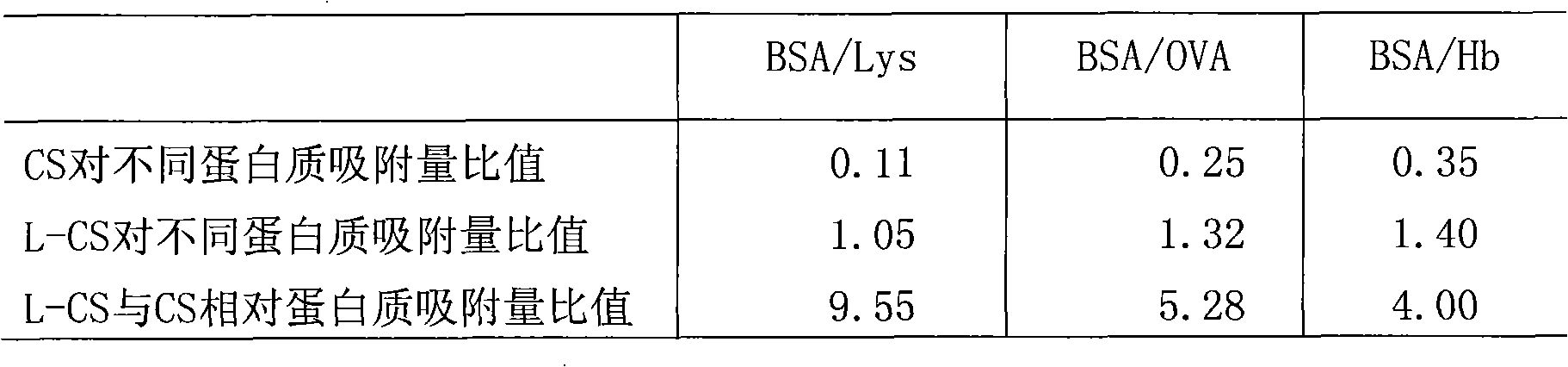
Method for preparing layer-by-layer self-assembled protein-imprinted polymer of chitosan
A technology of western imprinting and layer-by-layer self-assembly, which is applied in the fields of polymer chemistry and protein selective adsorption and separation, can solve the problems of complex preparation process, protein denaturation and inactivation, and achieve simple preparation method, reduced denaturation, and good protein selection The effect of adsorption and separation functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of layer-by-layer self-assembled western imprinted polymer of chitosan, it comprises the steps:
[0030] 1) Preparation of cross-linked chitosan spherical particle substrate:
[0031]① Weigh 1.5g of chitosan, add it to 50mL of 2% acetic acid solution by mass (2% by mass means 2g of acetic acid in 100g of acetic acid solution), stir until the chitosan is fully dissolved, and remove bubbles by ultrasonic Obtain the chitosan solution of 3mg / mL; Add dropwise in the 2mol / L sodium hydroxide solution of 2L with medical syringe (7# needle head) with the drop rate of 2mL / min, white spherical particle appears in the solution, wait until white After the spherical particles settled to the bottom, the white spherical particles were separated and washed repeatedly with deionized water for 6 times until the pH value of the solution reached 7; then separated to obtain spherical chitosan particles;
[0032] ② The separated chitosan particles were transferred to a c...
Embodiment 2
[0043] A preparation method of layer-by-layer self-assembled western imprinted polymer of chitosan, it comprises the steps:
[0044] 1) Preparation of cross-linked chitosan spherical particle substrate:
[0045] 1. Weigh 3.0g chitosan, add it to 100mL of 2% acetic acid solution by mass, stir until chitosan is fully dissolved, and ultrasonically remove air bubbles to obtain a 3mg / mL chitosan solution; then use a medical syringe ( 7# needle) was added dropwise to 2L of 2mol / L sodium hydroxide solution at a rate of 4mL / min, and white spherical particles appeared in the solution. After the white spherical particles settled to the bottom, the white spherical particles were separated and deionized Water was repeatedly washed 6 times until the pH value of the solution reached 7; then separated to obtain chitosan spherical particles;
[0046] ② The separated chitosan spherical particles were transferred to a conical flask filled with 150mL deionized water, and the pH value of the sol...
Embodiment 3
[0057] A preparation method of layer-by-layer self-assembled western imprinted polymer of chitosan, it comprises the steps:
[0058] 1) Preparation of cross-linked chitosan spherical particle substrate:
[0059] 1. Take by weighing 1.5g chitosan, join in the mass percentage of 50mL and be 2% acetic acid solution, after stirring until chitosan is fully dissolved, obtain the chitosan solution of 3mg / mL after ultrasonically removing air bubbles; Then use medical syringe ( 7# needle) was added dropwise to 1L of 2mol / L sodium hydroxide solution at a rate of 2mL / min, and white spherical particles appeared in the solution. After the white spherical particles settled to the bottom, the white spherical particles were separated and deionized Water was repeatedly washed 4 times until the pH value of the solution reached 7; then separated to obtain spherical chitosan particles;
[0060] ② The separated chitosan spherical particles were transferred to a conical flask filled with 50mL deio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com