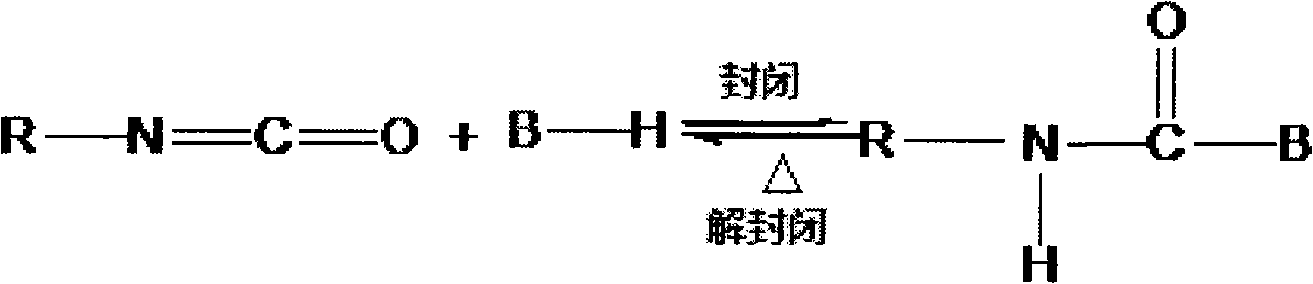Synthetic method of compound blocked polyisocyanates by using multiple sealants to compound and block terminal groups
A technology of polyisocyanate and synthesis method, which is applied in the field of composite polyisocyanate block synthesis, to achieve the effects of reasonable cost, improved performance, and improved stone chipping resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
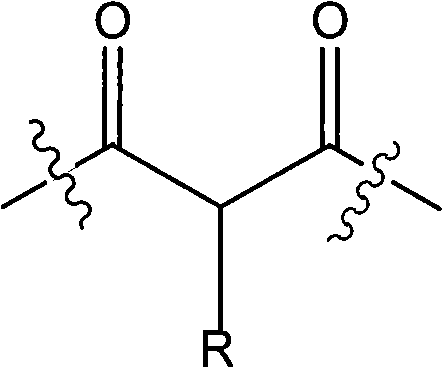
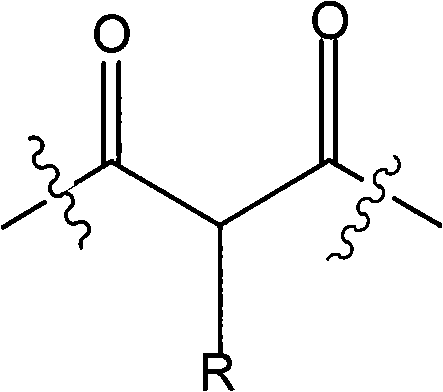
[0052] 468 grams of liquefied diphenylmethane-4,4'-diisocyanate, 160 grams of diethyl malonate, 133 grams of propylene glycol methyl ether acetate, 192 grams of naphtha 100 # Add the mixture to the container, stir at 70°C for about 2 hours; add 130 grams of ethyl acetoacetate, stir at 70°C for about 2 hours; add 109 grams of 3,5-dimethylpyrazole in batches, and stir at 80°C , and stir until no NCO groups are detected (about 1 hour).
[0053] The molar ratio of active methylene compound blocking agent (diethyl malonate and ethyl acetoacetate) to pyrazole blocking agent (3,5-dimethylpyrazole) is 2:1.
[0054] Compared with the prior art, the technical progress and advantages of the present invention are obvious, and it solves many problems that have plagued the synthesis of isocyanate blockers with a single blocking agent for a long time.
Embodiment 2
[0056] 386 grams of liquefied diphenylmethane-4,4'-diisocyanate (NCO% is 29%) and 147 grams of hexamethylene diisocyanate biuret, 80 grams of diethyl malonate, 142 grams of methyl isobutyl Base Ketone, 185g Naphtha 100 # Add the mixture to the container, stir for about 2 hours at 75°C; add 182 grams of ethyl acetoacetate, stir for about 2 hours at 75°C; add 120 grams of 3,5-dimethylpyrazole in batches, °C, stir until no NCO groups are detected (about 1 hour).
[0057] The molar ratio of the active methylene compound blocking agent (diethyl malonate and ethyl acetoacetate) to the pyrazole blocking agent (3,5-dimethylpyrazole) was 1.7:1.
[0058] Compared with the prior art, the invention has obvious technical progress and advantages, and solves many problems that have been perplexing the synthesis of isocyanate blockers with a single blocking agent for a long time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com