Coking oven gas hydrodesulfurization catalyst and method for preparing same
A hydrodesulfurization and catalyst technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. High temperature and other problems, to achieve the effect of uniform distribution of active components, high utilization rate and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0013] The preparation method of catalyst of the present invention comprises the following steps:
[0014] 1) Select the formed γ-Al 2 o 3 carrier, drying it at 120°C for 2 to 10 hours;
[0015] 2) Take a certain amount of ammonium molybdate and dissolve it in deionized water, add a certain amount of organic acid under constant stirring, then add cobalt salt, and finally add iron salt to obtain an impregnation solution; the organic acid can be Citric acid, lactic acid or tartaric acid, preferably citric acid. The cobalt salt and iron salt can be nitrate, carbonate or acetate, preferably nitrate. The molar ratio of Mo, Fe, Co and organic acid in the prepared immersion solution A is (14-55): (10-50): (0.5-24): (19-57).
[0016] 3) Immerse the carrier obtained in step 1) into the impregnating solution prepared in step 2) by equal impregnation method for 2 to 12 hours, then dry at 120°C, and then bake at 500°C for 2 to 4 hours to obtain Coke oven gas hydrodesulfurization cata...
Embodiment 1
[0026] 1) Weigh γ-Al dried at 120°C for 2 to 10 hours 2 o 3 Carrier 100g, spare;
[0027] 2) Weigh 24.5g of ammonium molybdate and dissolve it in 50ml of deionized water, add 30g of citric acid under constant stirring, then add 0.35g of cobalt nitrate, and finally add 25.3g of iron nitrate to prepare impregnation solution A, wherein Mo , Fe, Co, the molar ratio of organic acid is 55: 25: 2.4: 57;
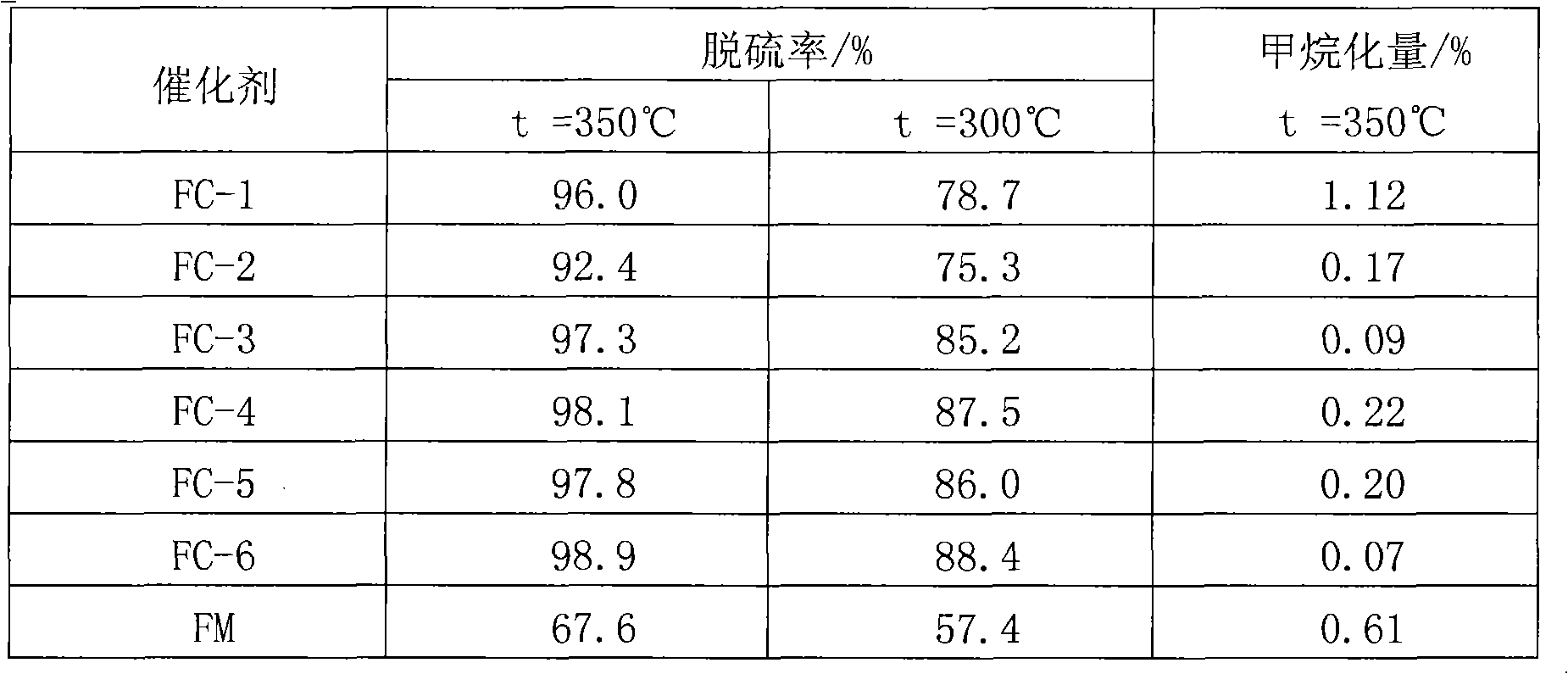
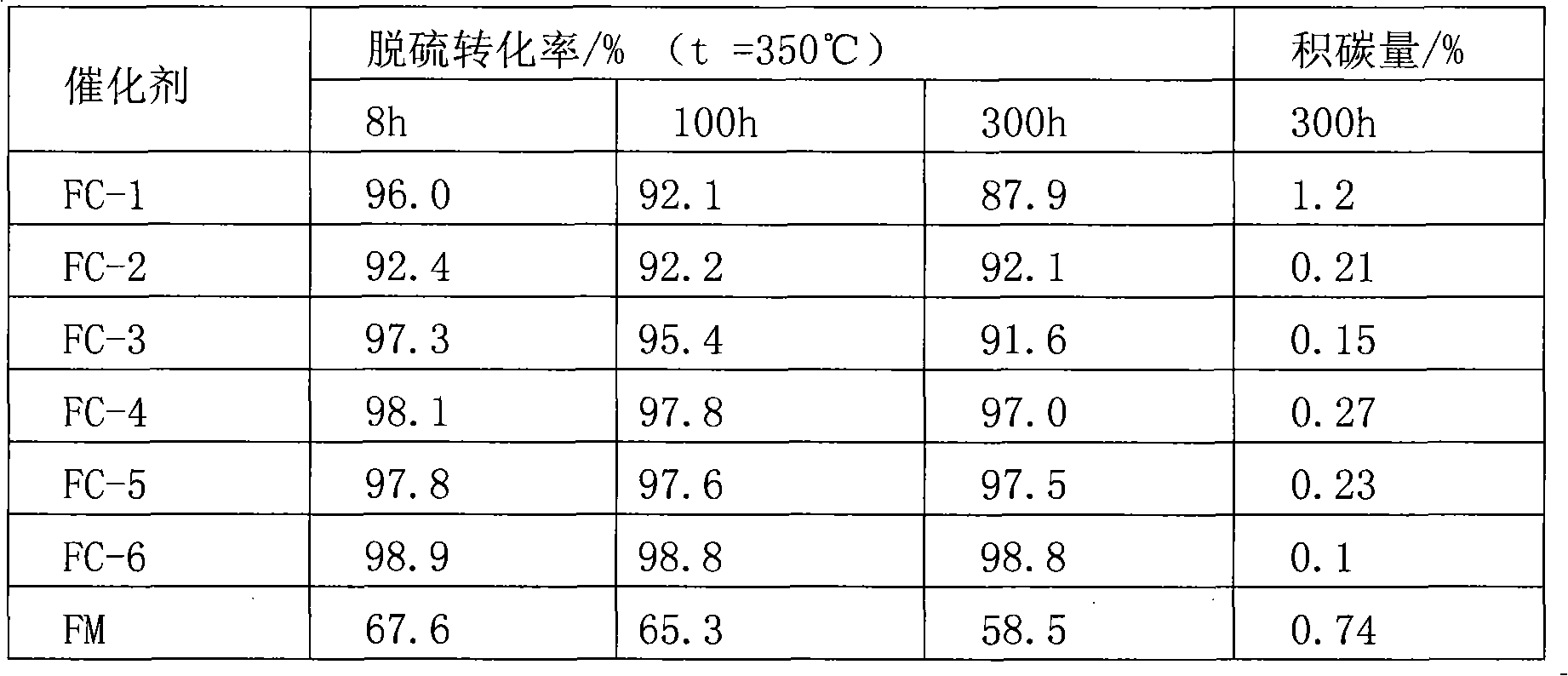
[0028] 3) Immerse the carrier obtained in step 1) into the impregnation solution A in step 2) for 2 to 12 hours, then dry at 120°C, and then roast at 500°C for 2 to 4 hours to obtain catalyst FC-1, The mass fractions of molybdenum oxides, iron oxides, and cobalt oxides are 20%, 5%, and 0.5%, respectively.
Embodiment 2
[0030] 1) Weigh γ-Al dried at 120°C for 2 to 10 hours 2 o 3 Carrier 100g, spare;
[0031] 2) Weigh 6.13g of ammonium molybdate and dissolve it in 50ml of deionized water, add 10g of citric acid under constant stirring, then add 0.35g of cobalt nitrate, and finally add 10.1g of iron nitrate to prepare impregnation solution A, wherein Mo , Fe, Co, the molar ratio of organic acid is 14: 10: 0.5: 19;
[0032] 3) impregnating the carrier obtained in step 1) into the impregnation solution A obtained in step 2) for 2 to 12 hours, then drying at 120°C, and then roasting at 500°C for 2 to 4 hours to obtain a catalyst precursor;
[0033] 4) Weigh 0.013g of cesium nitrate and add it to 60ml of deionized water to make impregnating solution B;
[0034] 5) Add the catalyst precursor obtained in step 3) into the impregnation solution B prepared in step 4) for immersion for 2 to 12 hours, then dry at 120°C, and then roast at 600°C for 2 to 4 hours to obtain Catalyst FC-2, wherein the mass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com