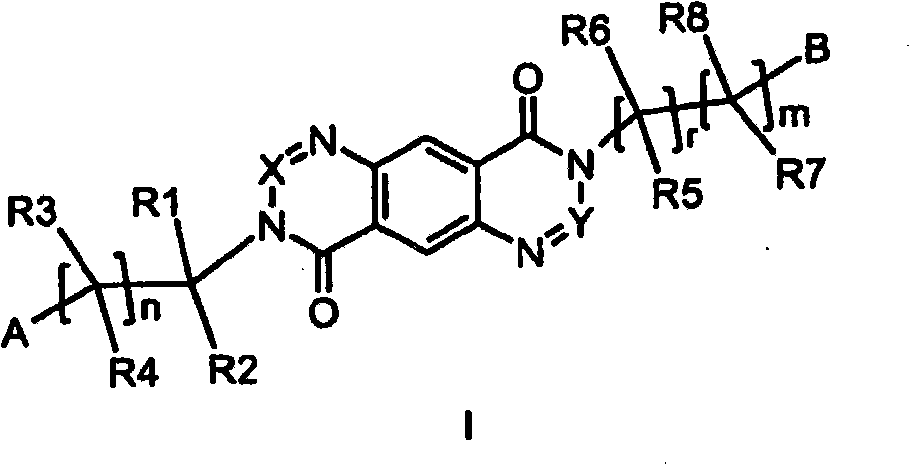
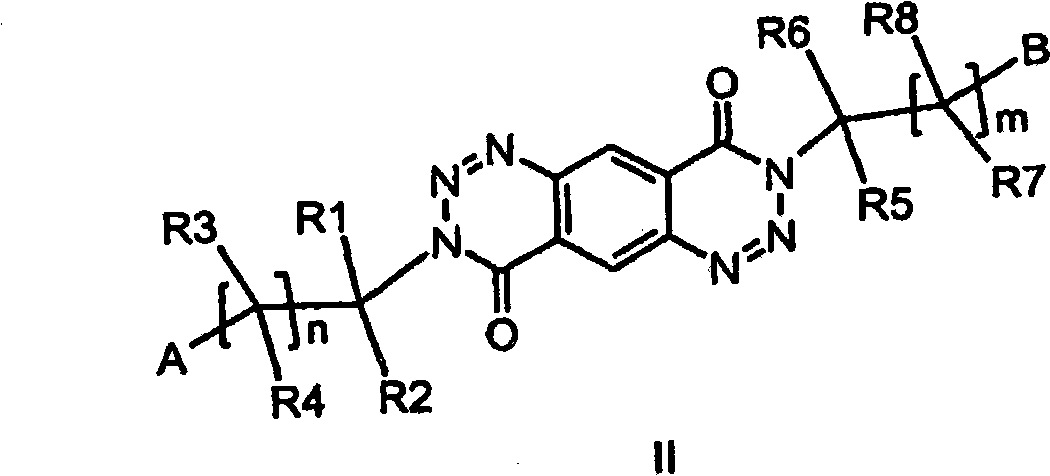
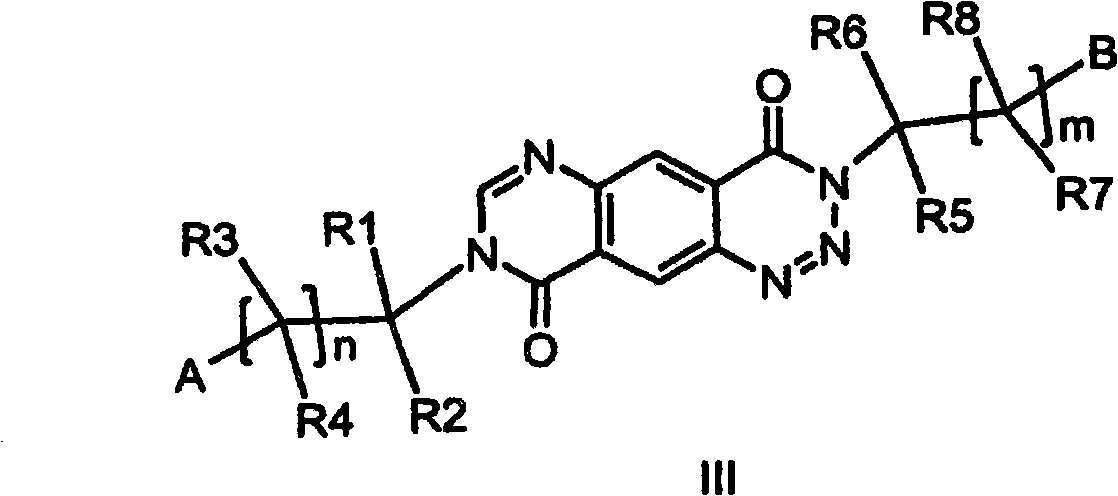
3-substituted 1,2,3-triazin-4-one's and 3-substituted 1,3-pyrimidinone's for enhancing glutamatergic synaptic responses
A CH2, C-H technology, applied in the field of compounds for the treatment of these pathological conditions, and the field of synaptic receptors, can solve the problems of uncertain mechanism of effect and low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0447] 3-Cyclopropyl-8-[(1R)-1-methyl-2-(2H-tetrazol-2-yl)ethyl]-3,8-dihydro[1,2,3]triazine [4,5-g][1,2,3]benzotriazine-4,9-dione
[0448]
[0449] Intermediates 7 and 8 (2.70 g, 9.8 mmol) were dissolved in THF (100 ml) and methanol (75 ml). A solution of potassium hydroxide (6.0 g) in water (60 ml) was added and the mixture was stirred (20°C) for 45 minutes. Hydrochloric acid was added to the mixture (to pH 2) and the solvent was evaporated. The mixture was dissolved in DMF (100ml) and the solvent was evaporated to remove water. (2R)-1-(2H-tetrazol-2-yl)propan-2-amine hydrochloride (2.0 g, 10 mmol) and DMF (100 ml) were added and the solvent was evaporated. Add DMF (80ml), dimethylaminopiperidine (DMAP) (1.22g, 10mmol), hydroxybenzotriazole (HOBT) (1.35g, 10mmol), NEt 3 (2ml) and N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide (EDCI) (5.5g, 28.5mmol) and the mixture was stirred at 45°C under nitrogen for 4 hours. Evaporate the solvent, add water (100ml), use 2N H 2...
Embodiment 2
[0451] 3-cyclopropyl-8-[(2R)-1-(5-methyl-2H-tetrazol2-yl)prop-2-yl]-3,8-dihydro[1,2-d:4 ,5-d'] Bis[1,2,3]triazine-4,9-dione
[0452]
[0453] The title compound was prepared from Intermediate 3 following the procedure of Example 1. The obtained white solid has the following characteristics: mp=157-160°C; 1 H NMR (300MHz, CDCl 3 )δ9.15(1H, s), 9.05(1H, s), 5.90-5.75(1H, m), 5.26(1H, dd, J=8.4 and 14.4Hz), 5.05(1H, dd, J=4.8 and 14.4 Hz), 4.12-4.02 (1H, m), 2.44 (3H, s), 1.79 (3H, d, J = 6.9 Hz) and 1.48-1.24 ppm (4H, m).
Embodiment 3
[0455] 3-cyclopropyl-8-[(2R)-1-(5-methyl-1H-tetrazol-1-yl)prop-2-yl]-3,8-dihydrobenzo [1,2-d:4,5-d']bis[1,2,3]triazine-4,9-dione
[0456]
[0457] Intermediate 4 was used to prepare the title compound following the procedure of Example 1. The obtained white solid has the following characteristics: mp=208-211°C; 1 H NMR (300MHz, CDCl 3 )δ9.14(1H, s), 9.02(1H, s), 5.90-5.79(1H, m), 5.03(1H, dd, J=9.6 and 14.4Hz), 4.69(1H, dd, J=5.1 and 14.4Hz), 4.11-4.03 (1H, m), 2.62 (3H, s), 1.80 (3H, d, J = 6.9Hz) and 1.45-1.25ppm (4H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com