Synthesis method of ethyl tetrahydrofurfuryl ether
A technology of ethyl tetrahydrofurfuryl ether and its synthesis method, which is applied in the direction of organic chemistry, can solve the problems of environmental pollution, high risk, and high cost, and achieve the effects of reducing pollution emissions, eliminating risk factors, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 500ML reactor, add 1mol tetrahydrofurfuryl alcohol, 1mol ethyl chloride, and 1mol potassium hydroxide, react under stirring, control the reaction temperature at 5°C, and finish the reaction after reacting for 9 hours, and distill the reaction product under atmospheric pressure. Fractions at 150°C to 160°C were collected to obtain 95g of the product.
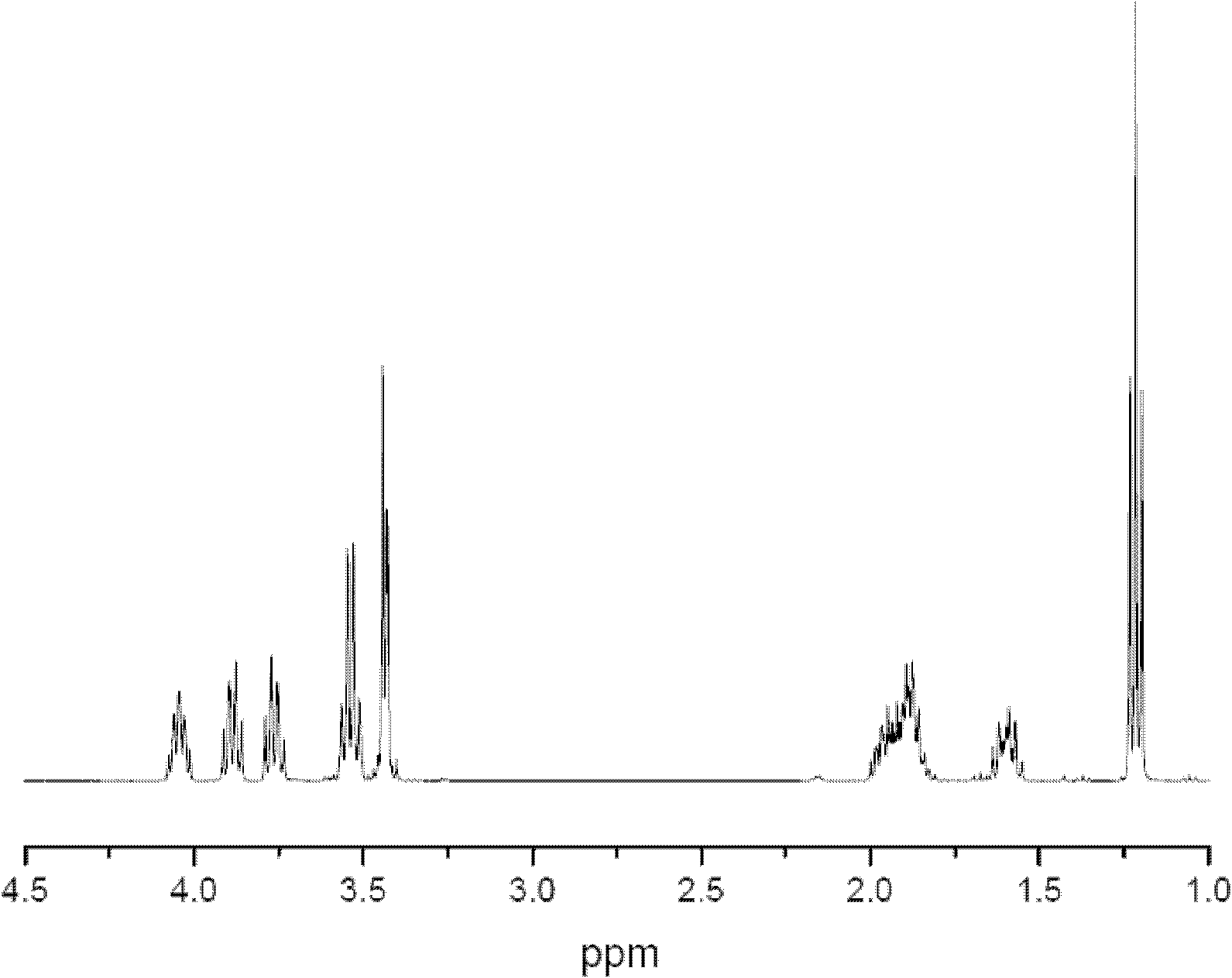
[0021] Using nuclear magnetic and infrared analysis, it was proved that the product was ethyl tetrahydrofurfuryl ether. Product adopts Bruker AVANCEDRX 400MHz nuclear magnetic resonance spectrometer to measure, and solvent is deuterated chloroform; The 1H-NMR resonance peak of its ETE belongs to as follows figure 1 As shown, the results are shown in Table 1.
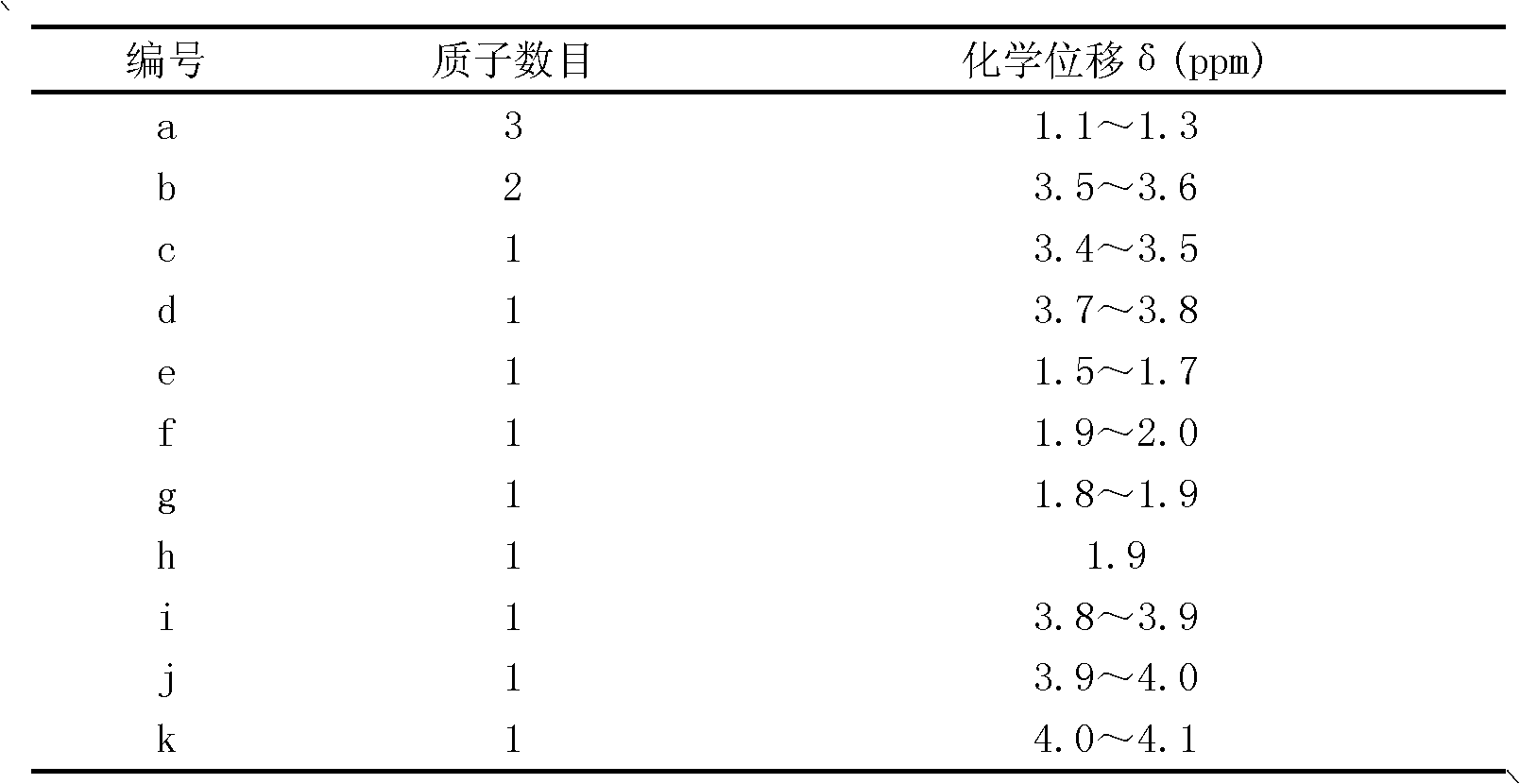
[0022] 1H-NMR resonance peak assignment of ETE in table 1
[0023]
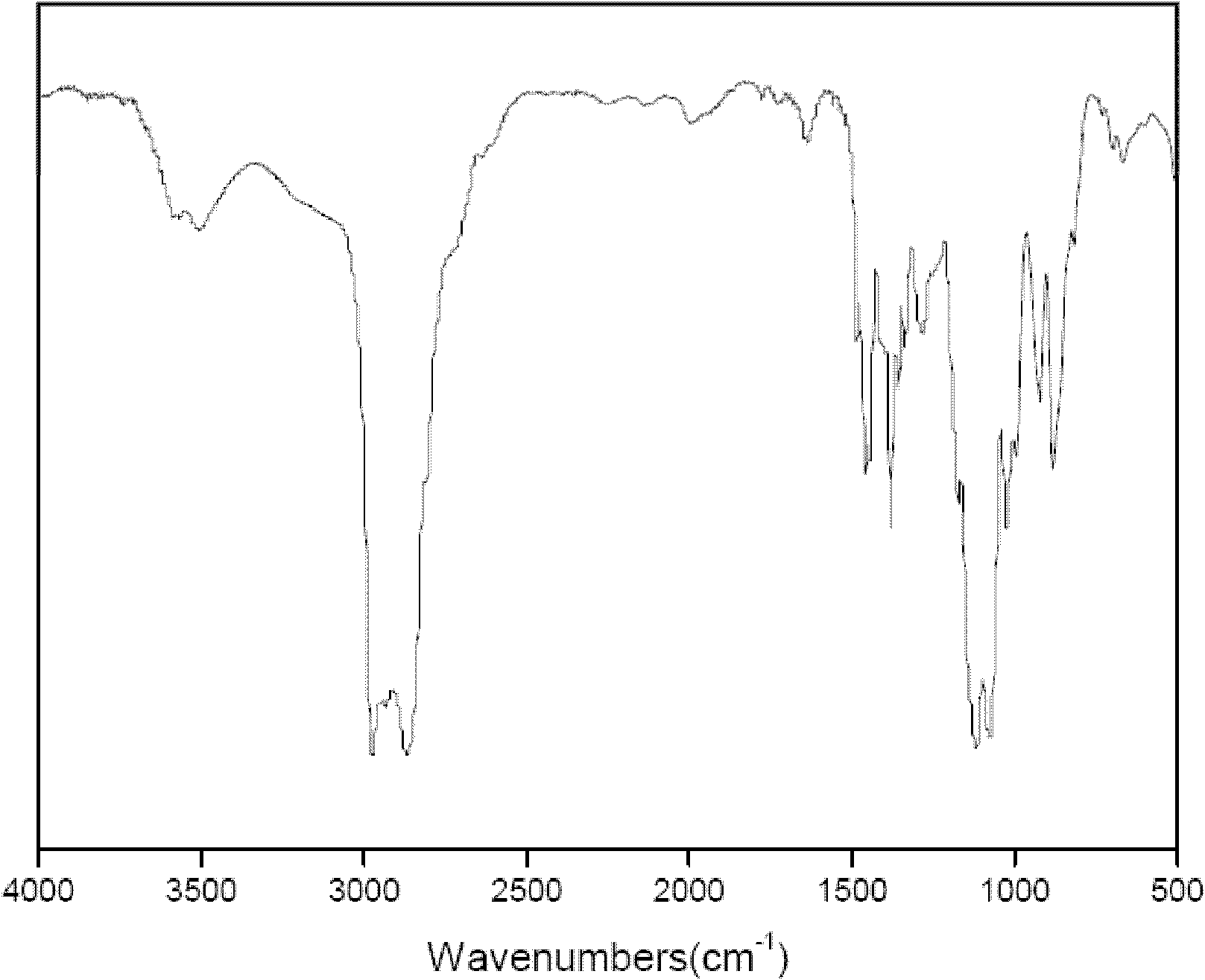
[0024] The structure of the target product is analyzed by a Nicolet 20DXB FT-IR infrared chromatograph, and the infrared spectrum is as follows figure 2 shown. Therefore, its molecular ...
Embodiment 2
[0028] In a 500ML reactor, add 1mol tetrahydrofurfuryl alcohol, 1.5mol ethyl chloride, and 1.5mol potassium hydroxide, react under stirring, control the reaction temperature at 5°C, and finish the reaction after reacting for 9 hours. For the product, the fraction at 150°C-160°C was collected to obtain 106g of the product. With nuclear magnetic and infrared analysis as shown in embodiment 1, prove to be ethyl tetrahydrofurfuryl ether; Molecular structure is:
[0029]
[0030] Analyzed by gas chromatography as shown in Example 1, the product purity is 98%; the yield of ethyl tetrahydrofurfuryl ether in tetrahydrofurfuryl alcohol is 80%.
Embodiment 3
[0032] In a 500ML reactor, add 1mol tetrahydrofurfuryl alcohol, 2mol ethyl chloride, and 2mol potassium hydroxide, react under stirring, control the reaction temperature at 50°C, and finish the reaction after 4 hours of reaction, and distill the reaction product under normal pressure. Fractions at 150°C to 160°C were collected to obtain 109g of the product. With nuclear magnetic and infrared analysis as shown in embodiment 1, prove to be ethyl tetrahydrofurfuryl ether; Molecular structure is:
[0033]
[0034] Analyzed by gas chromatography as shown in Example 1, the product purity was 98%; the yield of ethyl tetrahydrofurfuryl ether was 82% based on tetrahydrofurfuryl alcohol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com