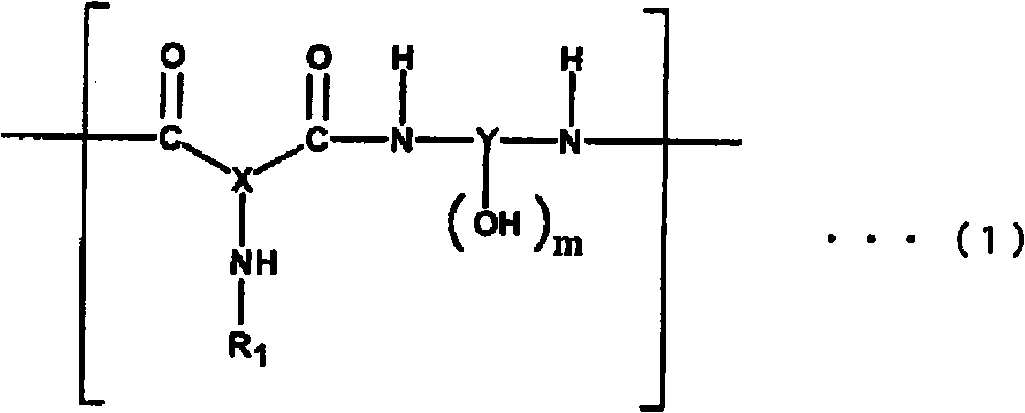
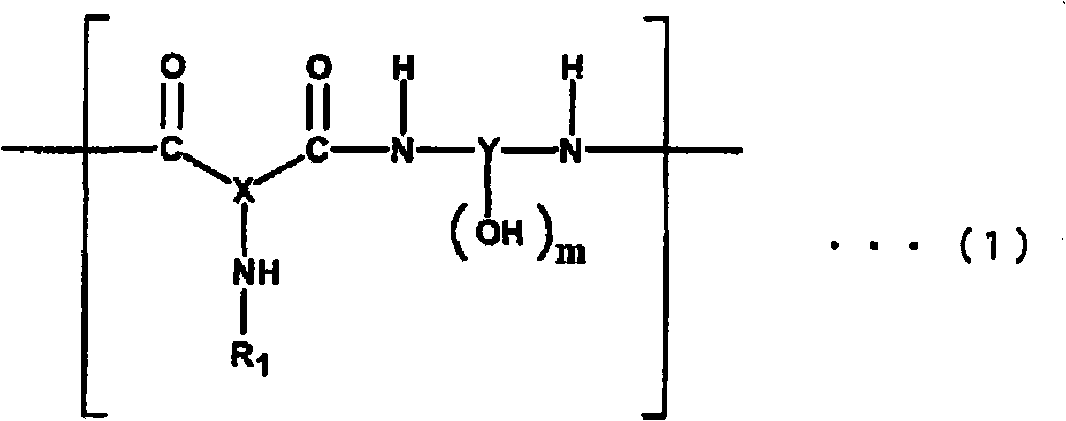
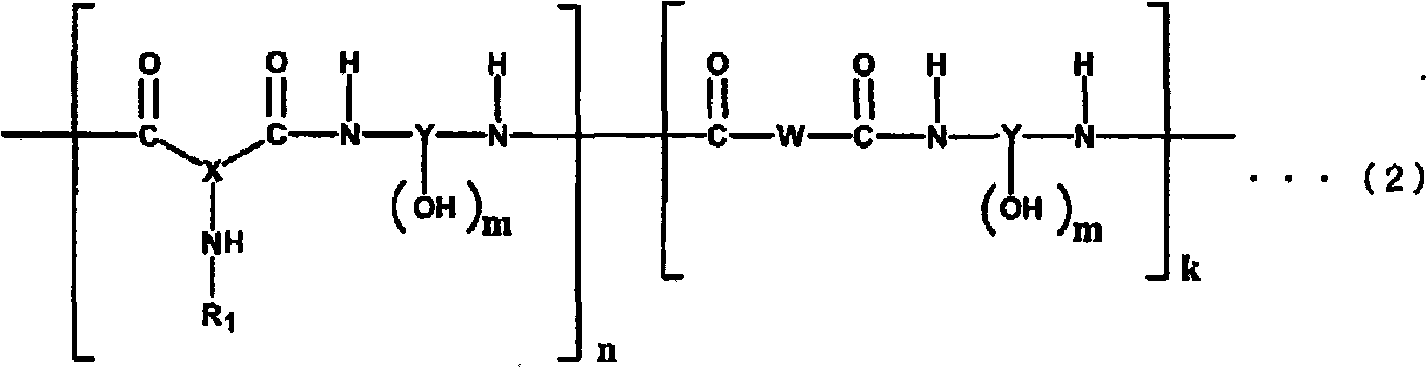
Polyamide resin, photosensitive resin composition, method for forming cured relief pattern, and semiconductor device
A technology of polyamide resin and photosensitive resin, which is applied in the fields of polyamide resin, photosensitive resin composition, formation of cured relief patterns and semiconductor devices, can solve the problem of inability to complete thermal imidization, deterioration of various physical properties, etc. problem, to achieve the effect of excellent chemical resistance and excellent chemical resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0196] Hereinafter, the present invention will be described by way of examples and comparative examples. In addition, the combination list of the polymer raw material of each synthesis example mentioned below is shown in the following Table 1.
Synthetic example 1
[0198] (Synthesis of phthalic acid compound sealer AIPA-MO)
[0199] Into a detachable flask with a capacity of 5 liters, 543.5 g (3.0 mol) of 5-aminoisophthalic acid {hereinafter abbreviated as AIPA was added. }, 1700 g of N-methyl-2-pyrrolidone {hereinafter referred to as NMP. }, mix and stir, and heat to 50°C in a water bath. 500 g of gamma-butyrolactone {hereinafter referred to as GBL was added dropwise thereto with a dropping funnel. } A solution obtained by diluting 512.0 g (3.3 mol) of 2-methacryloyloxyethyl isocyanate was stirred at 50° C. for about 2 hours as it was.
[0200]By low molecular weight gel permeation chromatography {hereinafter referred to as low molecular weight GPC. }After confirming that the reaction is complete (disappearance of 5-aminoisophthalic acid), the reaction solution is dropped into 15 liters of ion-exchanged water, stirred, left standing, waiting for the crystallization and precipitation of the reaction product, filtering, and after appropria...
Synthetic example 2
[0202] (Synthesis of phthalic acid compound block AIPA-BA)
[0203] In a detachable flask with a capacity of 5 liters, 543.5 g (3.0 mol) of AIPA and 1700 g of NMP were put in, mixed and stirred, and heated to 50° C. in a water bath. A solution obtained by diluting 789.46 g (3.3 mol) of 1,1-bis(acryloyloxymethyl)ethyl isocyanate with 500 g of GBL was added dropwise thereto with a dropping funnel, and stirred at 50° C. for about 2 hours as it was.
[0204] After confirming the completion of the reaction (disappearance of AIPA) by low-molecular-weight GPC, put the reaction solution into 15 liters of ion-exchanged water, stir and let it stand, wait for the crystallization and precipitation of the reaction product, filter it, wash it with water appropriately, and then place it at 40°C Vacuum drying for 48 hours, thereby obtaining AIPA-BA after the amino group of AIPA and the isocyanate group of 1,1-bis(acryloyloxymethyl)ethyl isocyanate reacted. The low molecular weight GPC purity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com