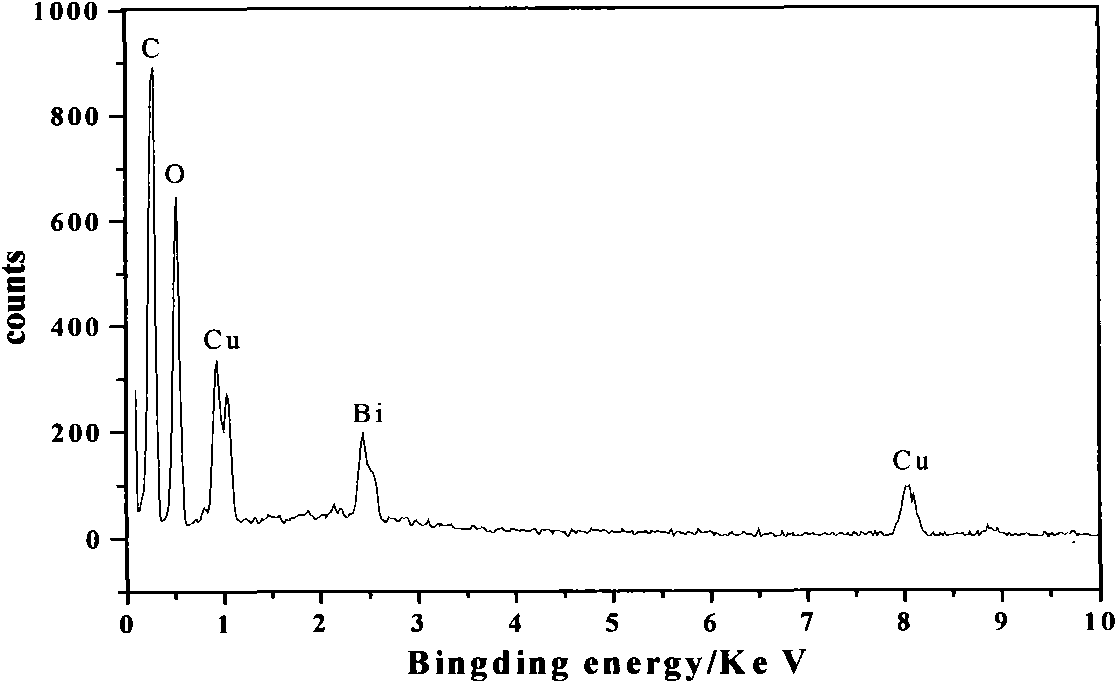
2, 3, 3', 4, 4', 5'-hexahydroxy benzophenone bismuth (III) and copper (II) binuclear complex and preparation method thereof
A kind of hexahydroxybenzophenone bismuth, hexahydroxybenzophenone technology, applied in 2,3,3',4,4',5'-hexahydroxybenzophenone bismuth (III) copper (II) In the field of binuclear complex and its preparation, it can solve problems such as harm and toxicity of lead compounds, and achieve high catalytic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) In a three-necked flask equipped with a stirrer and an HCl absorption system, add 3.38g gallic acid, 3.78g pyrogallic acid, 5g anhydrous ZnCl 2 and 6ml POCl 3 , and then add 3ml sulfolane.
[0018] (2) Start stirring to make it evenly mixed, heat up to 70° C., and react at constant temperature for 2.5 hours.
[0019] (3) Slowly add water into the reaction bottle and stir until it is completely dissolved, then pour into 300ml of ice water and stir well. The above solution was allowed to stand still for 30 minutes, filtered under normal pressure and washed with water to obtain a crude product.
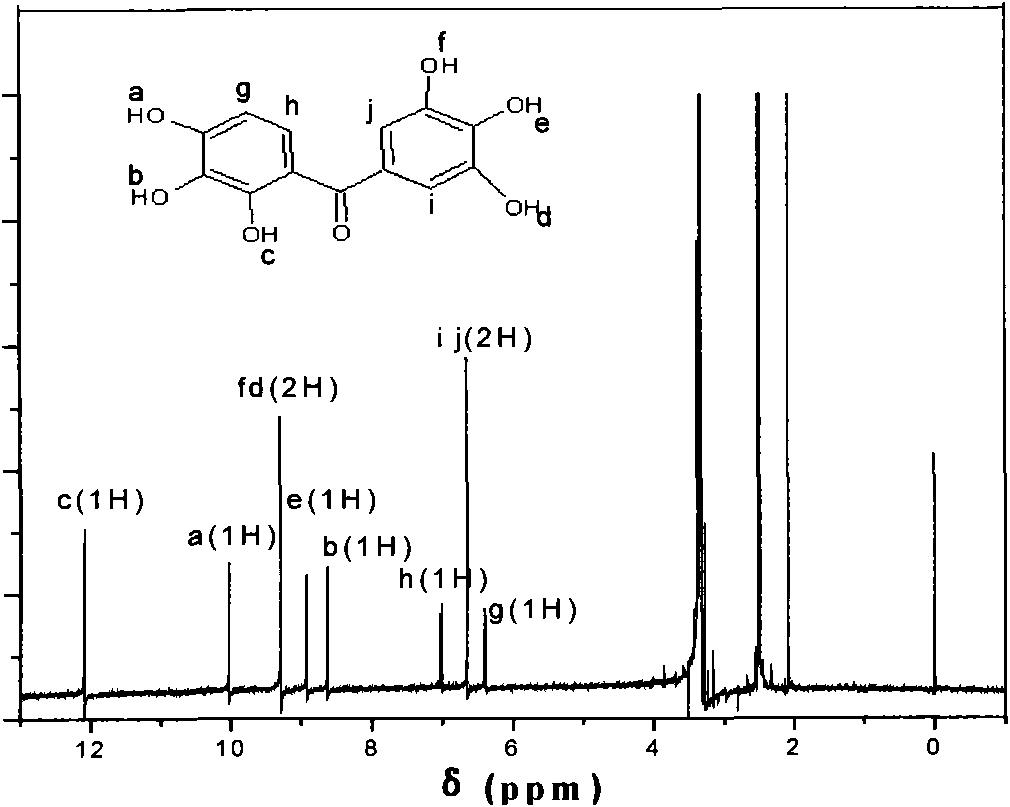
[0020] (4) Heat and dissolve the crude product in 200ml of water, add activated carbon, heat filter, cool, filter with suction, wash, dry, and obtain 2,3,3',4,4',5'-hexahydroxybenzidine The ketone is a green-yellow needle-like crystal with a melting point of 277.9-278.5°C and a yield of 68.5%. The product adopts FTIR spectrum and 1 HNMR spectrum to analyze its structure. ...
Embodiment 2
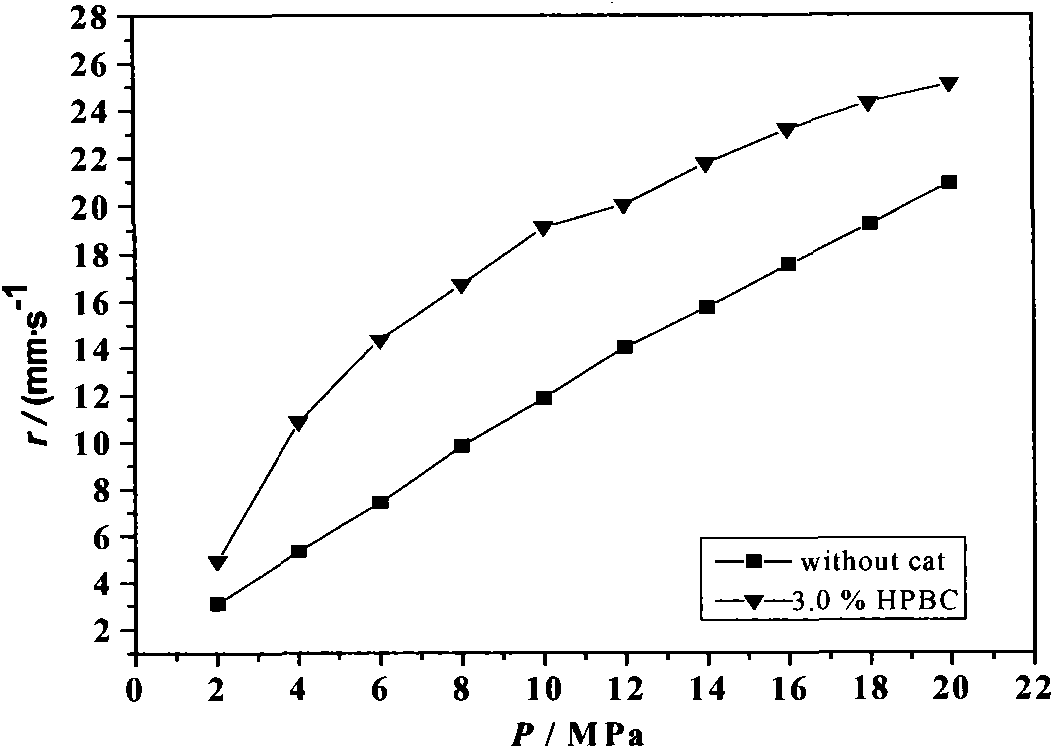
[0025] The basic formula of the modified double-base propellant is: double-base binder 66%, RDX 26%, No. 2 neutralizer (C 2 ) 2.0%, other additives 6%. The samples were prepared according to the conventional solvent-free compression molding process of absorption-water displacement-release-cooking-calendering-cutting into medicine strips. The medicine material is dosed according to 500g, and the catalyst is added in an amount of 3.0%; the blank propellant sample of the control group does not contain a catalyst.
[0026] The burning rate was measured by the target line method. The side of the treated Φ5×150mm small grain column was dipped and coated with polyvinyl alcohol solution for 6 times and dried in the air, and then the burning rate test was carried out in a slow-moving burning rate instrument filled with nitrogen. The test temperature is 20°C, and the pressure range is 2MPa~22MPa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com