Method for synthesizing thiodiglycol dimyristate
A technology of thiodiethylene glycol and myristate, applied in the preparation of thioether, organic chemistry, etc., can solve the problems such as no synthetic method of thiodiethylene glycol digyristate, and achieve good antioxidant properties , easy source, simple and feasible process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Thiodiethylene glycol is produced by Fengyuan Fine Chemical Company of Guangdong Zhonghe Chemical Plastic Co., Ltd., with a purity of 74.0%; analytically pure myristic acid is produced by Guangzhou Chemical Reagent Factory; the water-carrying agent is xylene; the catalyst is prepared by the following method Activated carbon loaded p-toluenesulfonic acid:
[0018] Soak the granular activated carbon washed with water and dried at 115°C in 100mL p-toluenesulfonic acid solution with a mass fraction of 25%, so that the load mass fraction of p-toluenesulfonic acid is 20%, filter, and filter the residue at 110°C Dry for 5h and set aside.
[0019] In reactor, add successively 15 moles of myristic acid, 10 moles of thiodiethylene glycol, and 1% catalyst of thiodiglycol and myristic acid gross mass and water-carrying agent by the thiodiglycol of 100 grams gross mass and myristic acid The metered amount is 30mL, stirred at a temperature of 170°C to 180°C until almost no water eva...
Embodiment 2
[0021] The difference from Example 1 is that the catalyst is concentrated sulfuric acid with a mass concentration of 75%.
Embodiment 3
[0023] The difference from Example 1 is that the catalyst is p-toluenesulfonic acid.
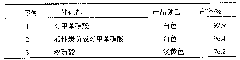
[0024] Table 1: Embodiment one, two and three adopt the influence of different catalysts on yield
[0025]
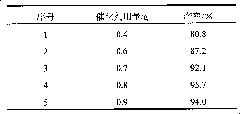
[0026] Table 2: the influence of catalyst consumption on productive rate (with embodiment one, just change catalyst consumption)
[0027]
[0028] Table 3: the influence of thiodiethylene glycol and myristic acid mol ratio on yield (with embodiment one, just change thiodiglycol and myristic acid mol ratio)
[0029]
[0030] Table 4: the influence of reaction time on productive rate (with embodiment one, just change changes reaction time)
[0031]
[0032] Product Structure Characterization:
[0033] Adopt Nicolet.760 infrared spectrometer, potassium bromide tablet method to measure the infrared spectrum of the product. The mass spectrum of the product was determined by Trace DSQGC / MS, and its melting point was determined on an XT-4 binocular melting point analyzer.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com