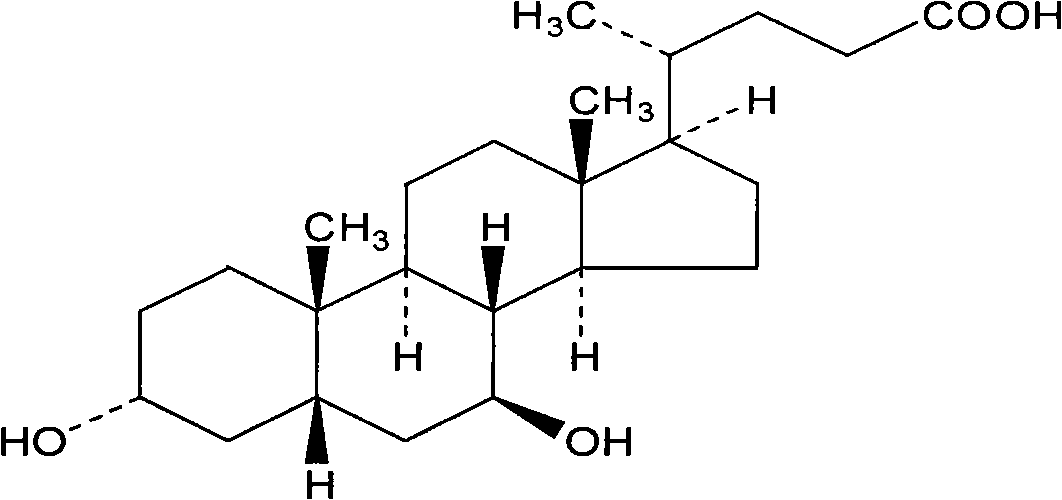
Ursodeoxycholic acid solid dispersoid, preparation method and solid preparation
A technology of ursodeoxycholic acid and solid dispersion, which is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pill delivery, etc. It can solve the problems of dissolution and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] (2) The preparation method of ursodeoxycholic acid solid dispersion
[0031] The preparation method of the ursodeoxycholic acid solid dispersion provided by the invention includes a melting method, a solvent method or a solvent melting method. Among them, the solvent method is preferable.
[0032] The melting method is to mix the drug and the carrier evenly (the carrier is coarsely powdered), heat it in a water bath or an oil bath until it melts, or heat the carrier to melt, then add a surfactant to melt, and finally add the drug to stir to dissolve, and then Under vigorous stirring, the melt was rapidly cooled into a solid.
[0033] The melting method according to the present invention comprises the following steps:
[0034] Take an appropriate amount of hydrophilic material, heat it in a water bath at 40-90°C to melt it, then add a surfactant, stir for 20-50 minutes to melt it, then add ursodeoxycholic acid, and stir again for 30-150 minutes , then pour out the mel...
Embodiment 1
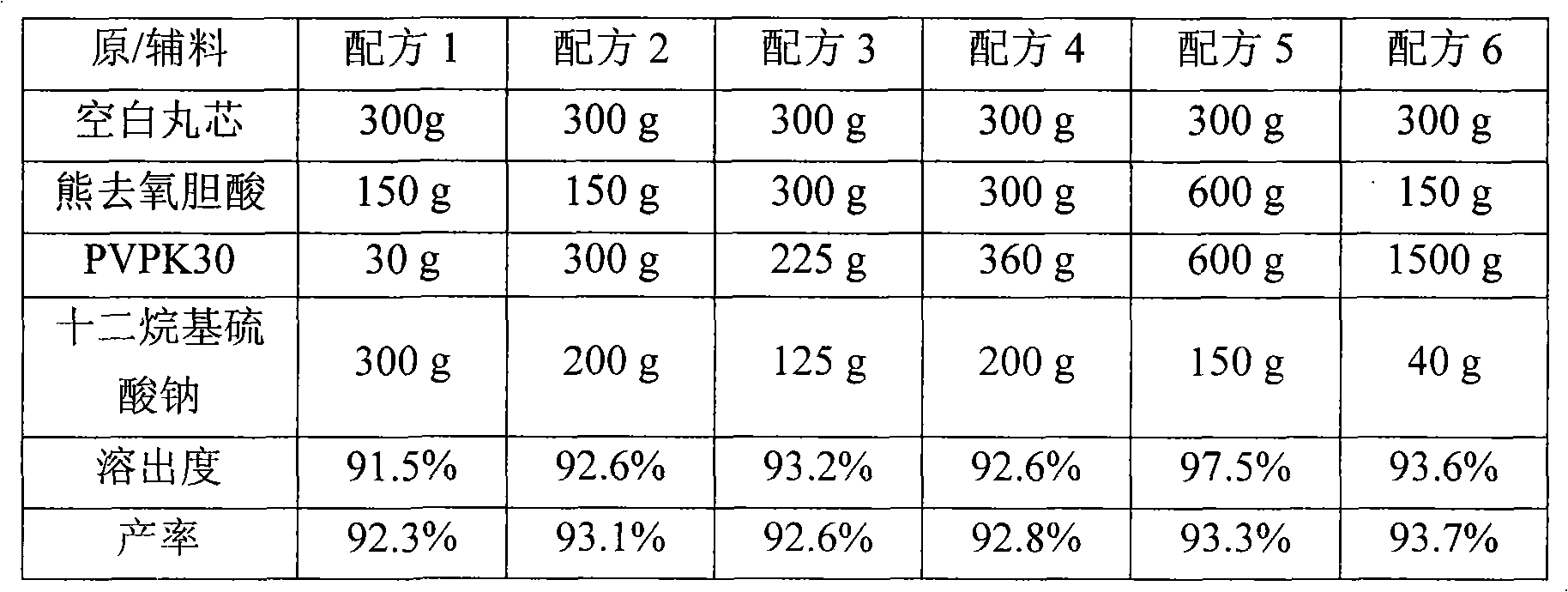
[0060] According to the formula shown in Table 1, put ursodeoxycholic acid and poloxamer into a beaker, add absolute ethanol, stir to dissolve completely, add sodium dodecylsulfonate, stir to dissolve. In a 40°C water bath, evaporate the solvent with a rotary evaporator for 35 minutes, and evaporate the solvent to dryness. Transfer to a vacuum drying oven to continue drying for 24 hours, take out, grind, and pass through an 80-mesh sieve to obtain a solid dispersion of ursodeoxycholic acid.
[0061] The above-mentioned ursodeoxycholic acid solid dispersion of the present invention is compressed into tablets to obtain the tablet of the present invention. The dissolution and productivity of the tablet of the present invention were measured, and the results are shown in Table 1.
[0062] Table 1
[0063] Raw materials
Embodiment 2
[0065] According to the formula shown in Table 2, the ursodeoxycholic acid was weighed and added into 90% ethanol, stirred and dissolved to a clear solution, and the formulated amount of PVPK30 was added, stirred and dissolved to obtain a clear solution.
[0066] Weigh the amount of sodium lauryl sulfate dissolved in water, stir to dissolve until the solution is clear.
[0067] The above two solutions are mixed to form a mixed solution or a homogeneous suspension.
[0068] The coating is carried out in a fluidized bed spray coating machine. After the machine is started, the blank ball core is placed in the fluidized bed coating machine, and under the action of hot air flow, it is in a suspended state. The above liquid medicine is transported to the fluidized bed by a peristaltic pump, sprayed and coated until all the liquid medicine is coated, then fluidized and dried in the fluidized bed. The parameters of the fluidized bed are as follows: bottom spray; fan frequency 20; mat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com