Anti-fatigue agent comprising amino acid composition
An anti-fatigue agent and amino acid technology, applied in the direction of anti-toxic agents, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of preparation trouble, cost increase, insufficient research, etc., and reduce the trouble of preparation and reduce the variety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4 and comparative example 1
[0136] Mice (male, 8-week-old, C57BL / 6N, feeding environment: 23±3°C, 12-hour light-dark cycle) were grouped as evenly as possible in each group, and divided into experimental groups consisting of 3 to 4 mice per group. Sample group (embodiment) and control group (comparative example). These mice were not fasted, but were forcibly orally administered the anti-fatigue agent of the present invention (sample) or physiological saline (control). One hour after the administration, an exercise load was applied by a treadmill test (25 m / min, 60 minutes).
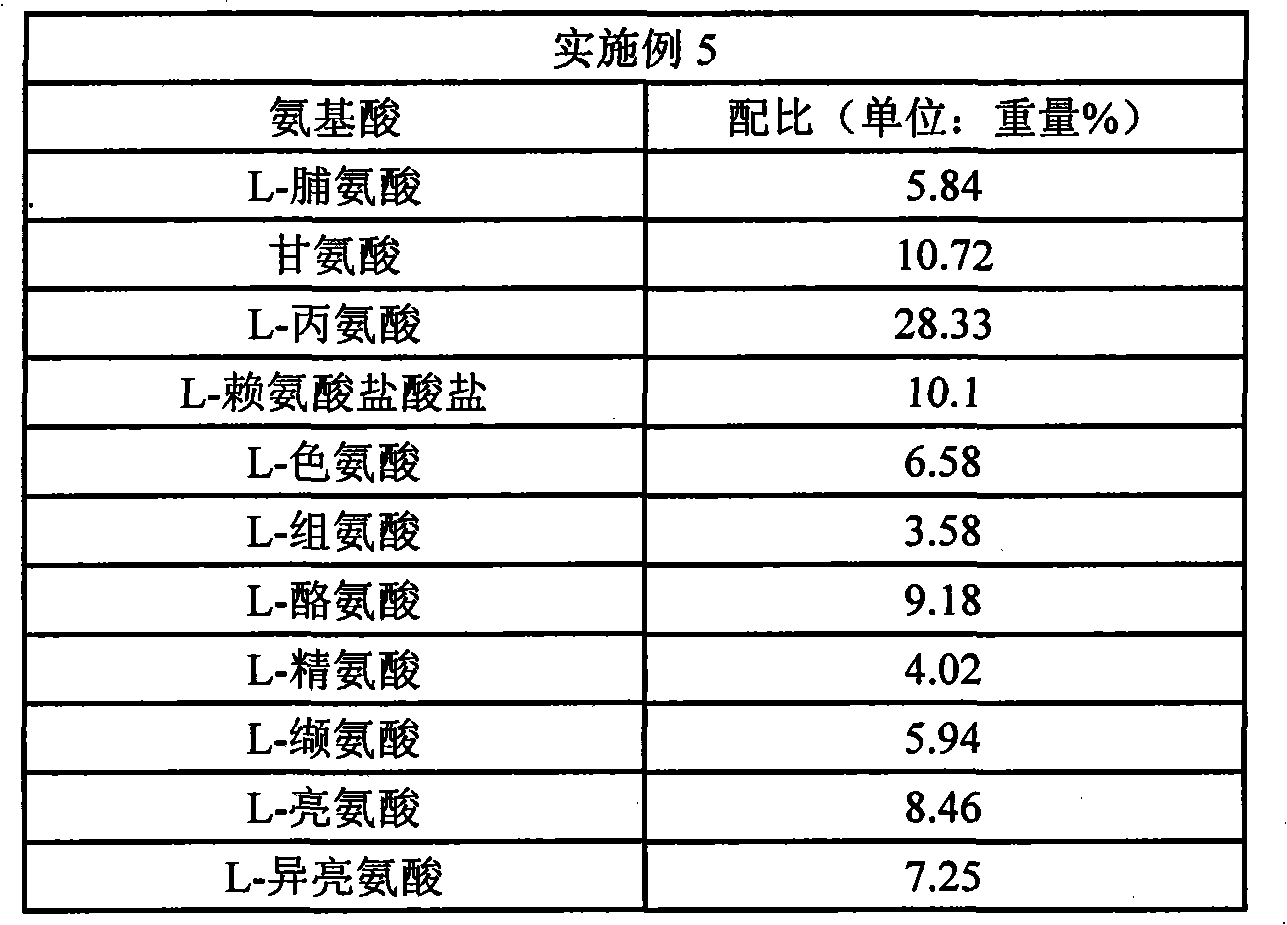
[0137] Each sample was administered to mice so as to achieve 500 mg / kg body weight in terms of amino acid (10 µL / g body weight for the suspension (5% by weight)). Here, the amino acid composition of the administered anti-fatigue agent of the present invention is shown in Table 1 (the numerical value of the amino acid composition is expressed in parts by weight).
[0138] (Test 1: Measurement of activity level when fatigued)
[01...
Embodiment 5
[0149] (Test 3: Anti-fatigue effect verification test)
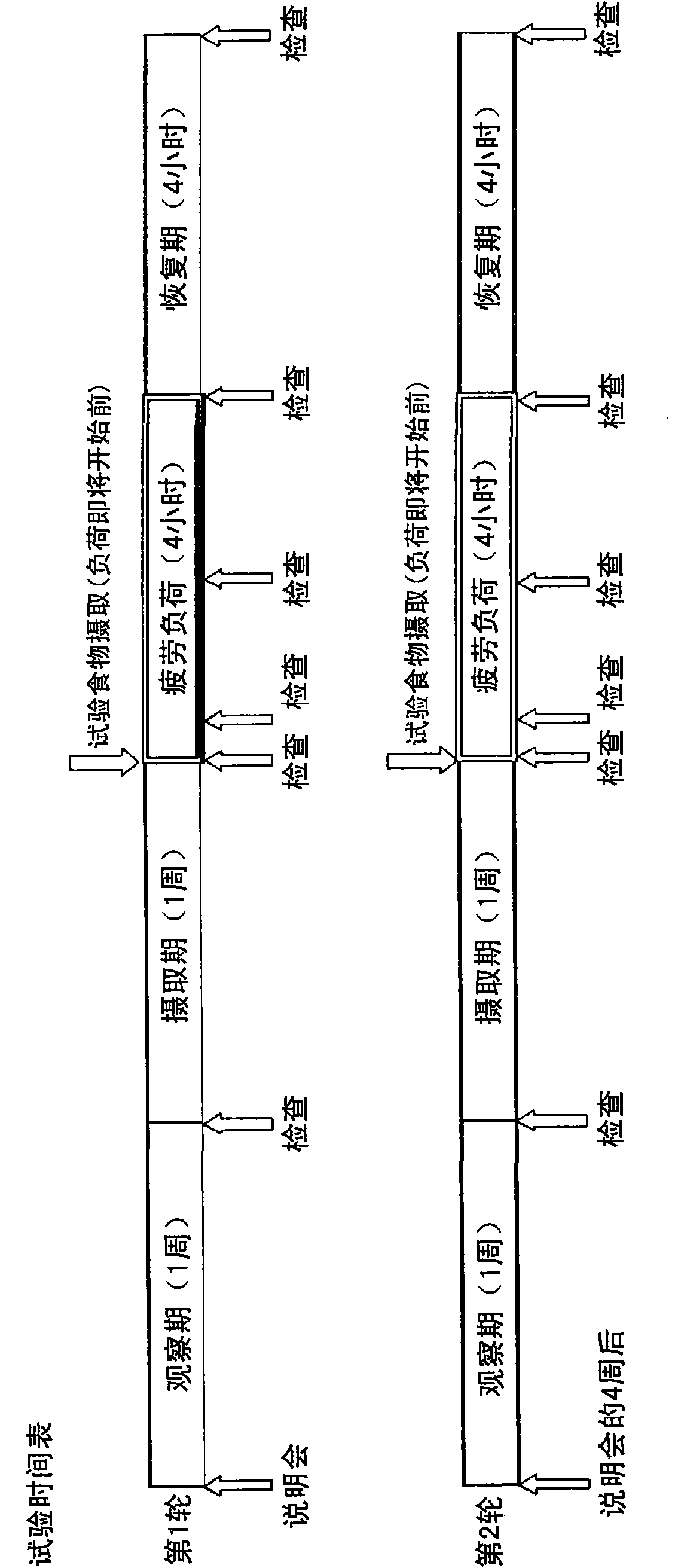
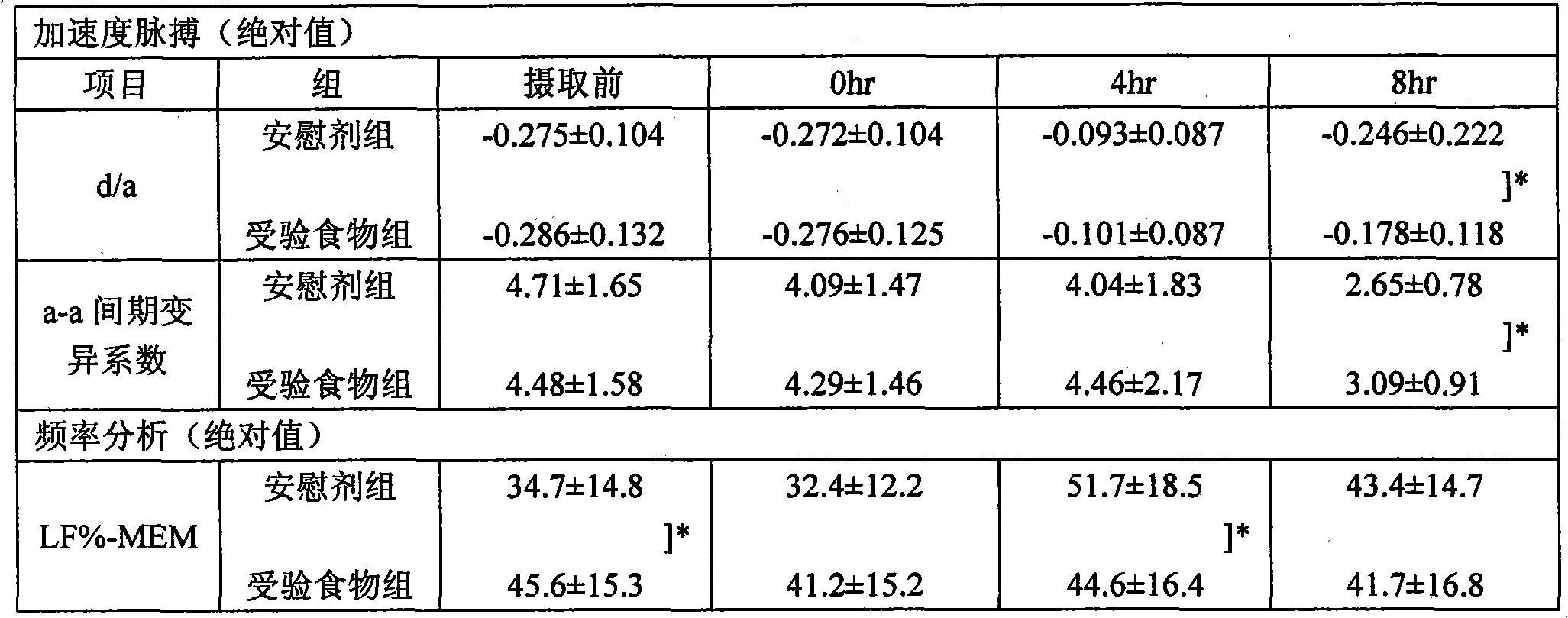
[0150] Next, human beings are subjected to an acceleration pulse test and a blood test that can be measured in a manner that does not impose unnecessary psychological and physical pain on the subject, and can objectively evaluate muscle fatigue and nerve fatigue during fatigue load. , urine test, saliva test and physical examination (physical exam), to further verify the anti-fatigue effect of the anti-fatigue agent of the present invention.
[0151] (1) Subjects
[0152] The subjects were healthy adult men and women (12 males, 6 females, 18 in total) who were judged by the physician in charge of the experiment to be suitable for participating in this experiment and agreed to participate in this experiment. This trial was carried out in accordance with the purpose of the Declaration of Helsinki on the basis of approval by the Institutional Review Board (IRB).
[0153] (2) Test food
[0154] The test food used in this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com