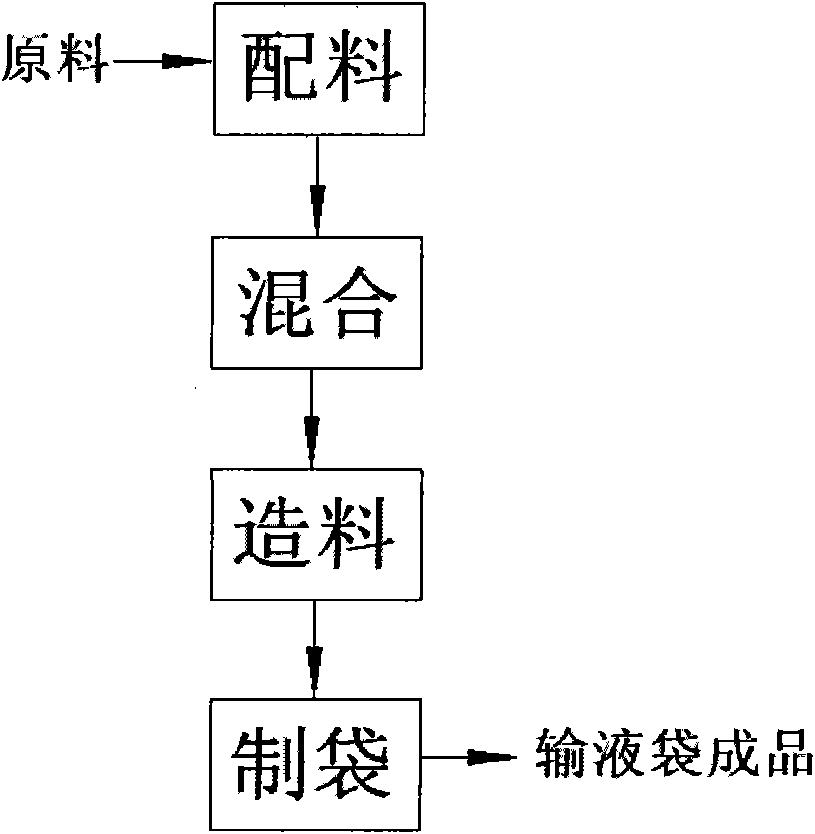
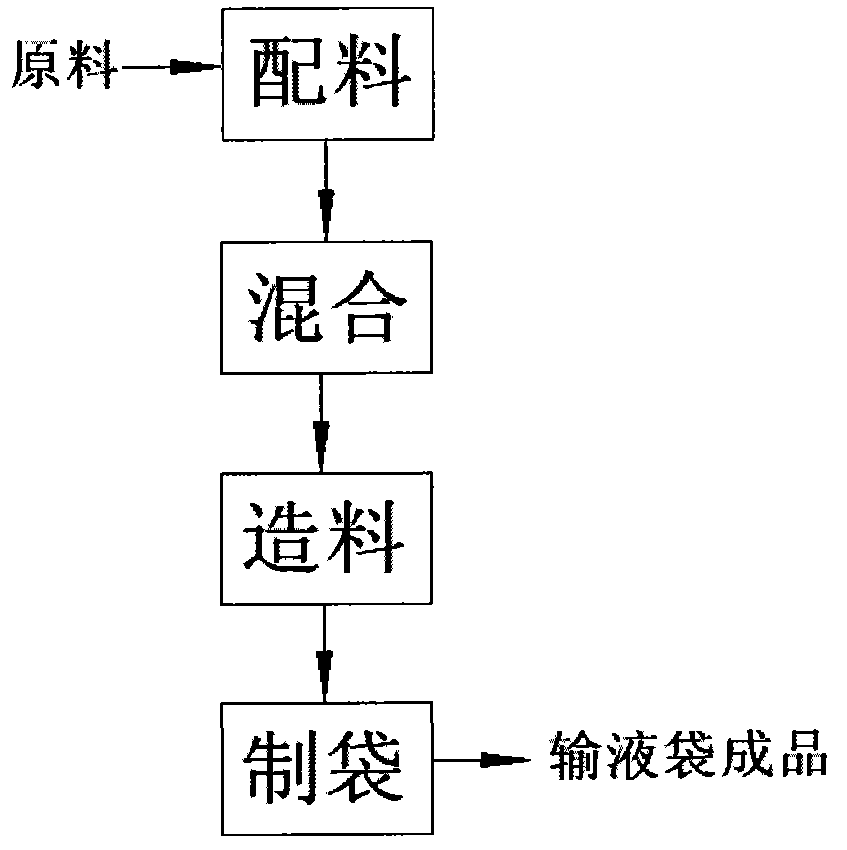
Manufacturing process and production equipment for medical infusion bags
A manufacturing process and production equipment technology, applied in transportation and packaging, medical containers, medical packaging, etc., can solve the problems of high resource and energy consumption, decreased safety and controllability, and inability to prevent leakage, etc., to achieve good transparency and compatibility Good performance, good heat sealing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] In the following, the present invention will be described in further detail through the specific description of the embodiments, so as to help those skilled in the art have a more complete, accurate and in-depth understanding of the inventive concepts and technical solutions of the present invention.
[0031] The first item of the present invention is a medical infusion bag. In the known technology, the material composition of the medical infusion bag mainly includes polypropylene (PP).
[0032] Polypropylene (PP) is the main component of medical infusion bags. Polypropylene (PP) has good chemical stability, including resistance to strong acids, strong alkalis and most organic substances. Polypropylene (PP) has excellent air tightness and vapor barrier properties, and is especially suitable for making infusion products that require high-temperature sterilization.
[0033] The medical transfusion bag of the present invention actually includes two different schemes for s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| brittleness temperature | aaaaa | aaaaa |
| service temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com