Application of ginsenoside Rb3 in preparing medicines for treating depressive disorder
A technology of ginsenoside and depression, which is applied in the field of medicine to achieve the effect of making full use of resources, reducing waste, and avoiding treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
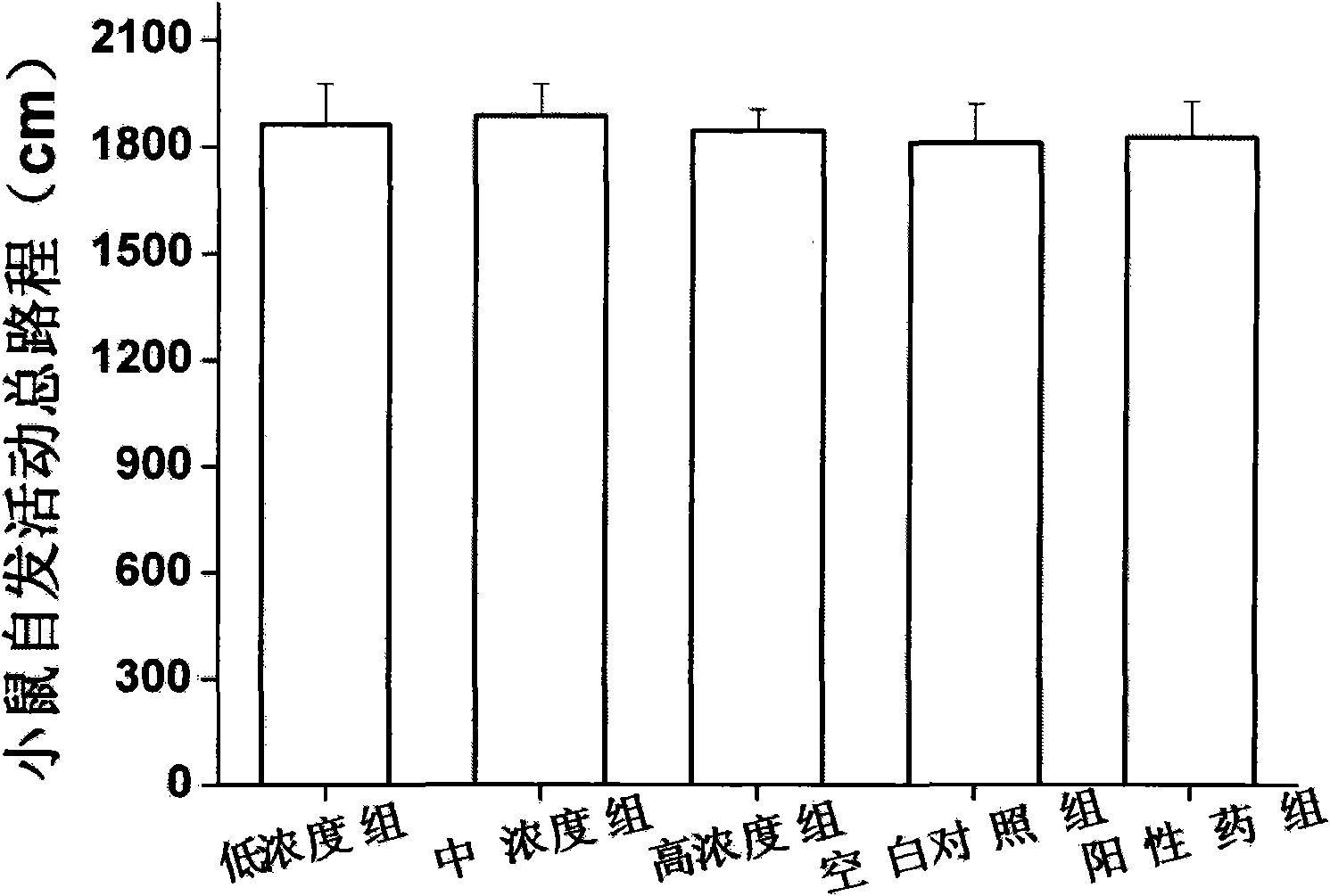
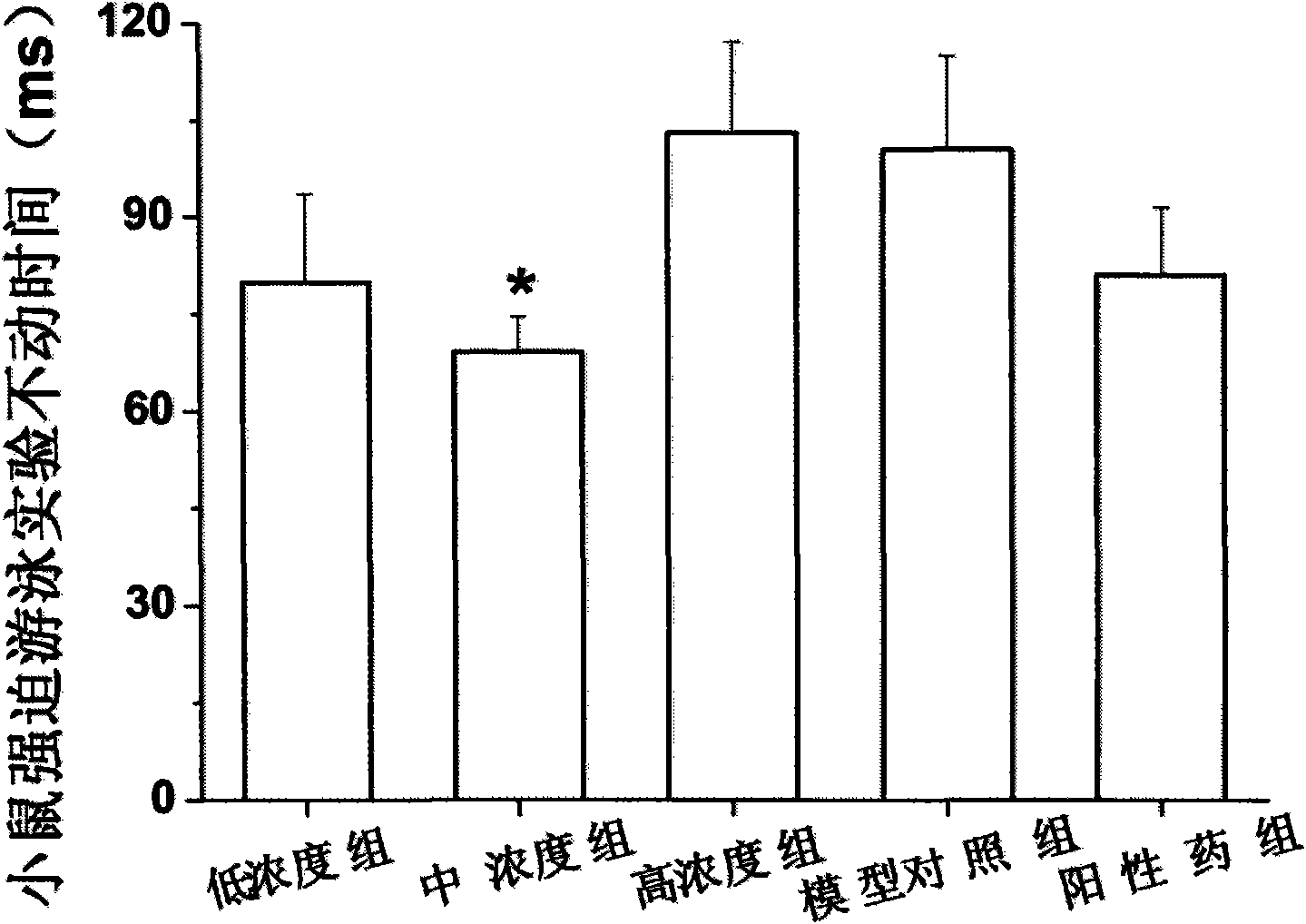
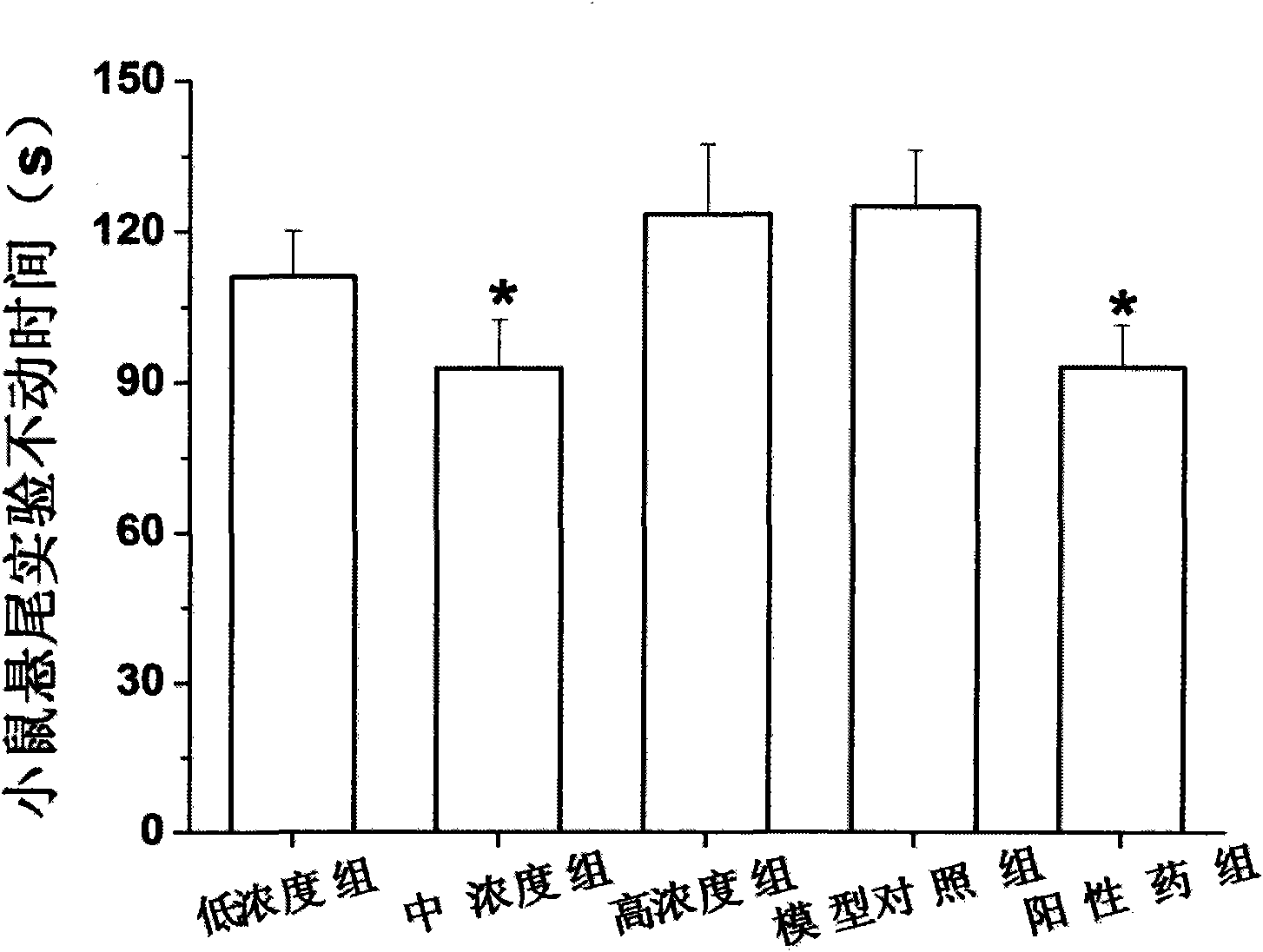
[0031] Example 1 Mice Behavior Despair Experiment Determination of Antidepressant Activity of Ginsenoside Rb3
[0032] Two models of behavioral hopelessness and depression were used, namely the mouse forced swimming model and the tail suspension model. Determination of time to explore the antidepressant effect of ginsenoside Rb3. In addition, before the establishment of the behavioral hopelessness model, the determination of the locomotor activity of the mice was performed to rule out the possibility of false positive results due to increased excitability.
[0033] Among them, the open-field measurement experiment of the spontaneous activity of mice, the forced swimming experiment and the tail suspension experiment were all carried out according to the general pharmacological method.
[0034] Select male NIH mice, SPF grade, 18-20 g, purchased from the Guangdong Provincial Experimental Animal Center, license number: SCXK (Guangdong) 2008-0002; they were randomly divided into ...
Embodiment 2
[0041] Example 2 Determination of antidepressant activity of ginsenoside Rb3 by mouse acquired helplessness experiment
[0042] The acquired helplessness experiment in mice used in this example was carried out according to general pharmacological methods.
[0043]Male NIH mice, SPF grade, 18-20 g, were purchased from Guangdong Experimental Animal Center, license number: SCXK (Guangdong) 2008-0002; they were randomly divided into 6 groups, with at least 10 mice in each group, and pressed once a day. 0.1ml / 10g body weight orally administered. Wherein the blank control group (No Inescapable Shock, NIS), the model control group (Inescapable Shock, IS) mice were given distilled water, and the administration group was respectively high concentration ginsenoside Rb3 group (IS+150mg / kg Rb3), medium concentration ginsenoside Rb3 group (IS+75mg / kg Rb3), low concentration ginsenoside Rb3 group (IS+30mg / kg Rb3), positive drug fluoxetine hydrochloride group (IS+10mg / kgFluooxetine). In th...
Embodiment 3
[0046] Example 3 Determination of antidepressant activity of ginsenoside Rb3 by mouse chronic mild unpredictable stimulation model experiment
[0047] This example establishes a chronic mild unpredictable stress model in mice according to general pharmacological methods, and on this basis, explores the effects of ginsenosides on the basis of the determination of mouse body weight, autonomous activity, sugar water preference rate, and novelty-inhibited feeding latency. The antidepressant effect of Rb3, and through the determination of the proportion of hippocampus and the relative content of BDNF in the prefrontal cortex and hippocampus of mice in each group, its mechanism of action was analyzed.
[0048] Male NIH mice, SPF grade, 18-20 g, were purchased from the Guangdong Provincial Experimental Animal Center, license number: SCXK (Guangdong) 2008-0002; except for 7 blank control groups, the rest of the mice were housed in single cages. Do model building. The modeling process...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com