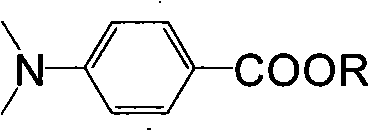
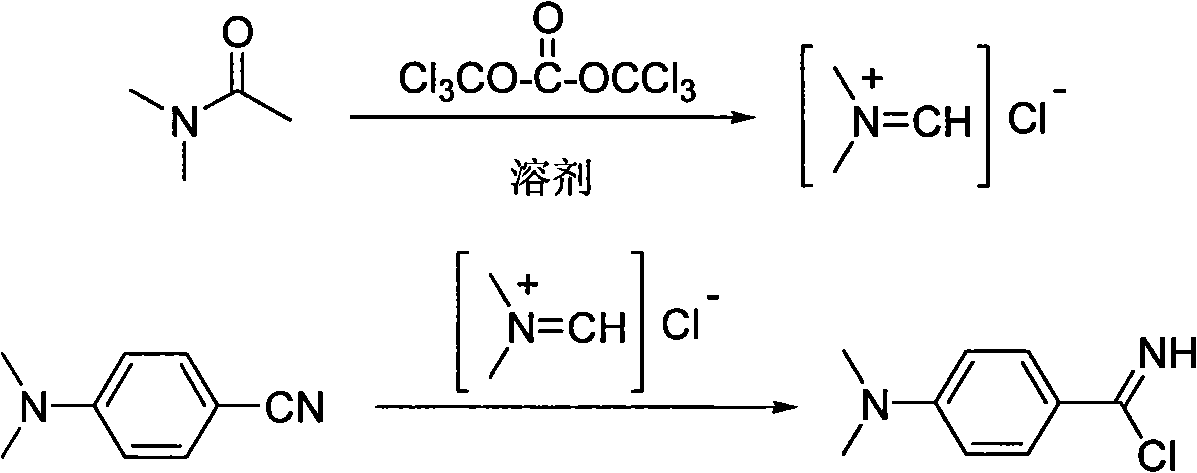
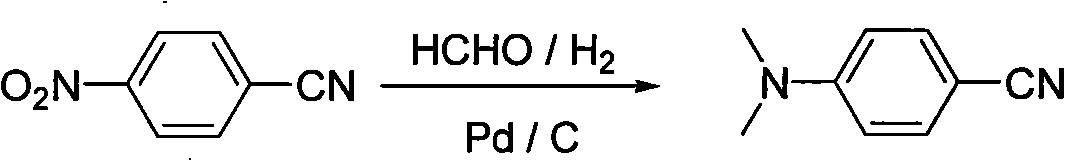Method for synthesizing ultraviolet photoinitiator of p-dimethylamin benzoic ether compounds
A technology of dimethylaminobenzoic acid and ester compounds, which is applied in the field of synthesis of p-dimethylaminobenzoic acid ester compounds as an ultraviolet photoinitiator, and can solve problems such as low esterification yield, affecting yield, and large amount of acidic wastewater. problem, to achieve the effect of moderate length of synthetic route, solvent recovery and application, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] step one:
[0031] (1) Preparation of p-dimethylaminobenzonitrile
[0032] In a 1L autoclave, add 74.0g (0.5mol) p-nitrobenzonitrile, 100ml methanol, 90g formaldehyde (wt 40%) and 10g concentrated sulfuric acid, then add 3.7g Pd / C (wt 5%) to seal the reaction The system was replaced with nitrogen and hydrogen 3 times successively. After stirring for 15 minutes, the temperature was raised to 60°C. Hydrogen was passed through to maintain the hydrogen pressure at 0.5MPa. The hydrogen absorption rate was regularly measured. When no hydrogen was absorbed for 30 minutes, the reaction was stopped. Cool to room temperature and release the pressure, filter the reaction solution to recover Pd / C, neutralize to neutral with 20% aqueous sodium bicarbonate solution, add 150ml of toluene for extraction, stand to separate the organic layer, and recover the solvent by distillation under reduced pressure, the obtained crude product is further 65.7g of the product was obtained by rectifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com