Transgenic chickens with an inactivated endogenous gene locus
A transgenic chicken, endogenous gene technology, applied in genetic engineering, peptides, immunoglobulins, etc., can solve the problems of expression destruction, inability to excise positive selection cassettes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1. Production of chicken PGC cultures
[0088]Incubate 2-5 μL of blood from the sinus terminalis of 14-17 stage (H&H) embryos in culture medium containing stem cell factor (SCF; 6 ng / ml or 60 ng / ml) in a 96-well plate , human recombinant fibroblast growth factor (hrFGF; 4ng / ml or 40ng / ml), 10% fetal bovine serum and 80% KO-DMEM conditioned medium. Preferably, 1-3 μL is taken from the vasculature of stage 15-16 (H&H) embryos. STO cells treated with irradiation were treated with 3 × 10 4 cells / cm 2 Inoculate the wells of a 96-well plate.
[0089] By adding 10% fetal bovine serum, 1% penicillin / streptomycin, 2mM glutamine, 1mM pyruvate, 1× nucleosides, 1× non-essential amino acids and 0.1mM β-mercaptoethanol and containing 5% fetal bovine KO-DMEM conditioned medium was prepared by culturing BRL cells in DMEM with serum for 3 days until confluent. After 24 hours, the medium was removed and a new batch of medium was conditioned for 3 days. This was repeated 3 t...
Embodiment 2
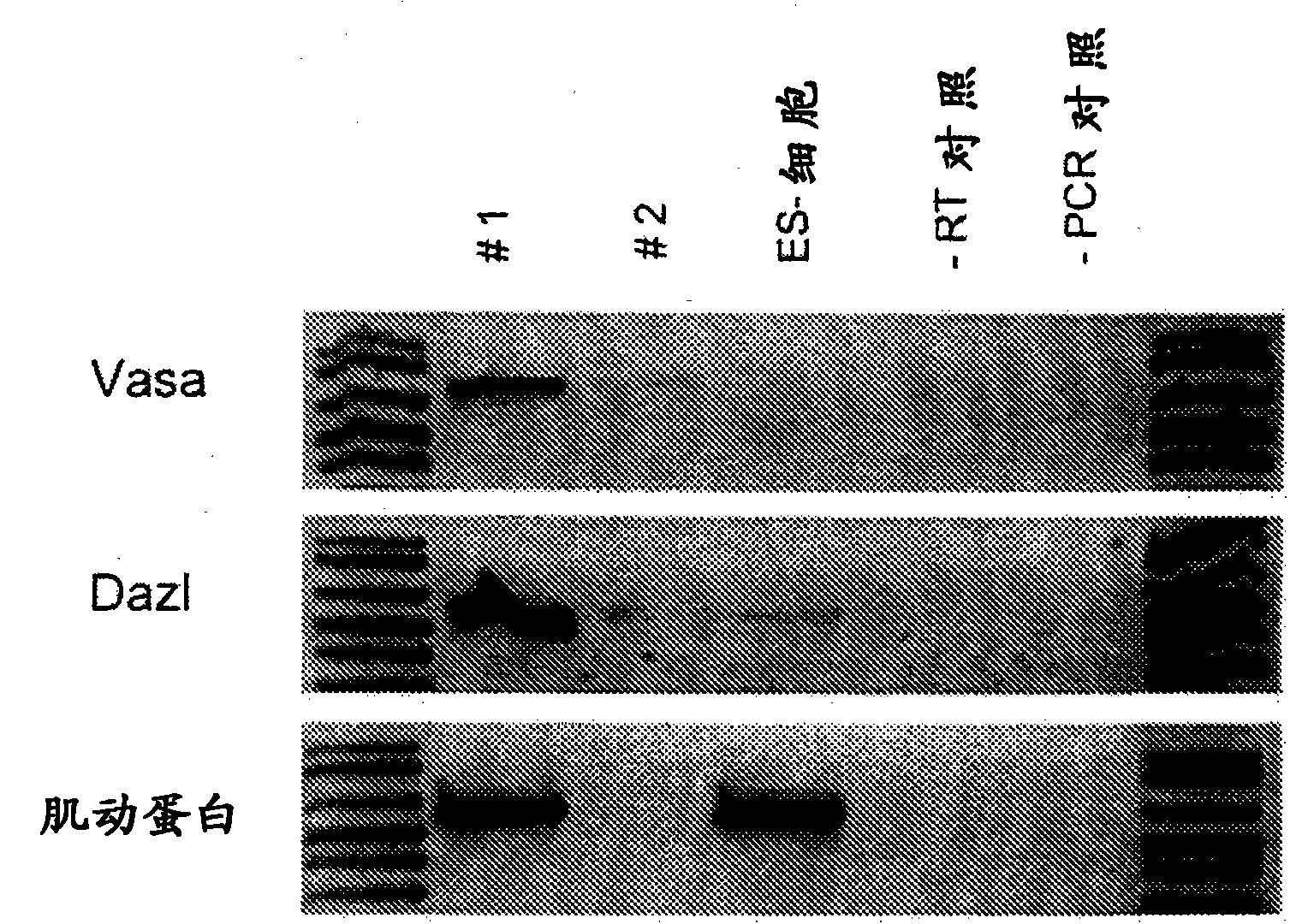
[0093] Example 2. Cultured PGCs express CVH and Dazl
[0094] Expression of CVH (the chicken homologue of the germline-specific gene VASA in Drosophila) is restricted to cells in the chicken germline and is expressed by about 200 cells in the germline crescent. (Tsuuekawa, N., Naito, M., Sakai, Y., Nishida, T. & Noce, T. Isolation of chicken vasahomolog gene and tracing the origin of primordial germ cells. Development 127, 2741-50. (2000)). In males, CVH expression is required for normal germline function, and loss of CVH function renders male mice infertile. (Tanaka, S.S. et al. The mousehomo log of Drosophila Vasa is required for the development of male germcells. Genes Dev 14, 841-53. (2000). In frog (Houston, D.W. & King, M.L.Acritical role for Xdazl, a germ plasm- localized RNA, in the differentiation of primordial germ cells in Xenopus. Development 127, 447-56, 2000), salamander (Johnson, A.D., Bachvarova, R.F., Drum, M. & Masi, T. Expression of axolotl DAZL RNA, a mark...
Embodiment 3
[0103] Example 3. PGC expresses CVH protein
[0104] Proteins were extracted from freshly isolated PGCs using the T-Per Tissue Protein Extraction Kit (Pierce). Pass at 1% NP 4 Cells were lysed to extract protein from cells in O; 0.4% deoxycholated 66 mM EDTA; 10 mM, Tris, pH 7.4. Samples were electrophoresed on 4-15% Tris-HCl precast gels (Bio-Rad). After transfer to the membrane, Western blotting was performed using the SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce) as indicated in the instruction manual. Rabbit anti-CVH antibody was used as primary antibody (1:300 dilution) and HPR-conjugated goat anti-rabbit IgG antibody (Pierce, 1:100,000) was used as secondary antibody ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com