Method for preparing Ni/MgO-Al2O3 catalyst for coproduction of methylisobutylketone and diisobutylketone
A technology of methyl isobutyl ketone and diisobutyl ketone, applied in the field of synthesizing methyl isobutyl ketone, can solve the problems of long process flow, difficult catalyst regeneration, large investment, etc., and achieve simple preparation process, suitable for The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
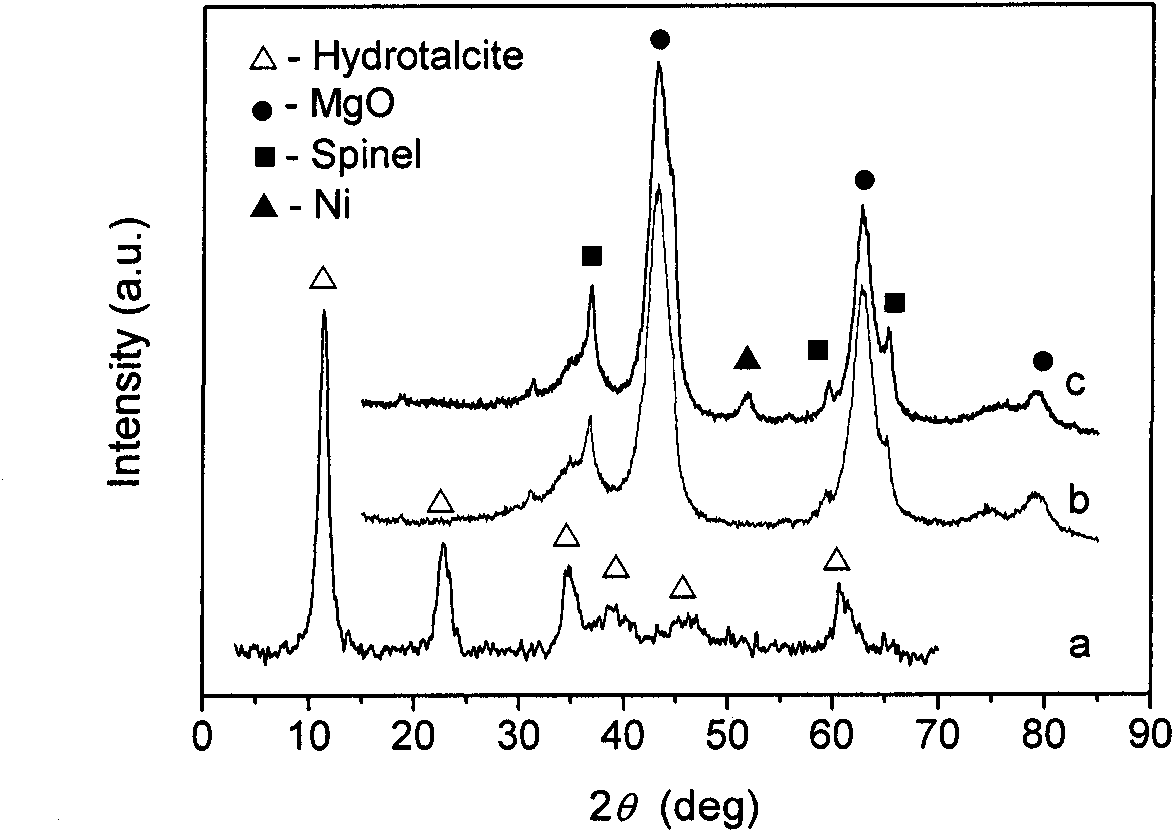
[0018] Use freshly boiled distilled water to prepare 1.0mol / L Mg(NO 3 ) 2 , Al(NO 3 ) 3 Metal salt solution; prepare another 1.0mol / L NaOH solution and 0.5mol / L NaOH solution 2 CO 3 solution. According to the Mg / Al molar ratio of 2:1, the nitrate solution of Mg and Al was made into a mixed solution, and the solution was designated as A. A certain amount of Na was added to the beaker in advance 2 CO 3 Solution [CO 3 2- The molar weight of = 0.5 (Al 3+ +Zr 4+ ) molar weight], solution A and NaOH solution are simultaneously titrated into the beaker, the rate of addition is controlled, and stirred to keep the pH between 9 and 10. After the dropwise addition, stir and age in a constant temperature water bath at 60°C for 12 hours, wash and centrifuge until the pH is around 7, perform suction filtration, put the filter cake in an oven, and dry at 80°C for 12 hours to obtain MgAl water Talc compound. The prepared MgAl hydrotalcite precursor was placed in a muffle furnace ...
Embodiment 2
[0021] The catalyst is the same as in Example 1, the reaction temperature is 180° C., the molar ratio of hydrogen to ketone is 1, and the reaction is carried out under different liquid space velocities. The results are shown in attached table 2.
Embodiment 3
[0023] Catalyst is the same as embodiment 1, and reaction temperature is 180 ℃, and liquid space velocity is 4.8ml / g cat h, react under different hydroketone molar ratios, the results are shown in attached table 3.
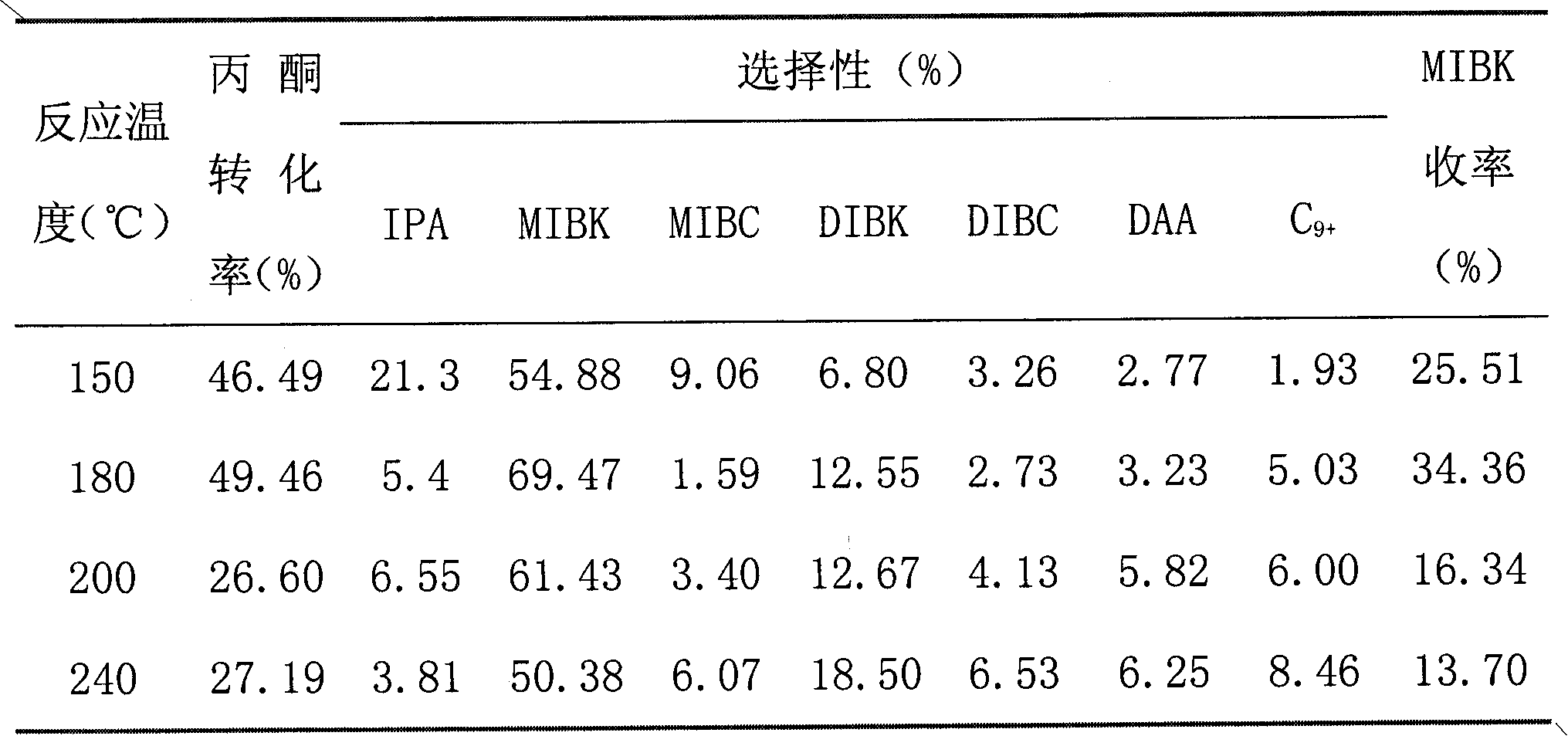
[0024] Attached Table 1 Catalytic performance at different reaction temperatures
[0025]
[0026] Note: IPA-isopropanol, MIBC-methyl isobutyl carbinol, DIBC-diisobutyl carbinol, DIBK-diisobutyl ketone, DAA-diacetone alcohol, C 9+ - Isophorone, mesitylene and other high-boiling compounds containing 9 carbons or more.
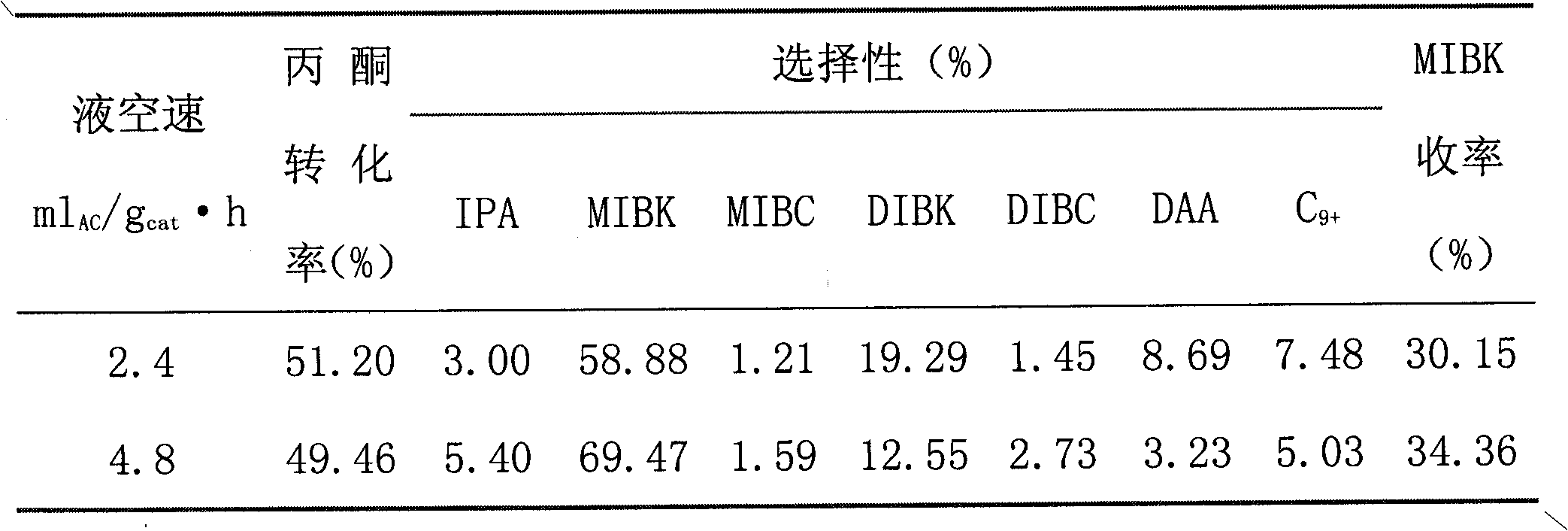
[0027] Attached Table 2 Catalytic performance at different liquid space velocities
[0028]
[0029]
[0030] Note: Please refer to Attached Table 1 for symbols in the table.
[0031] Schedule 3 Different H 2 / Catalytic performance under ketone
[0032]
[0033] Note: Please refer to Attached Table 1 for symbols in the table.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com