Polymer capable of performing addition reaction with thiol and having stable bonding hydrolysis
A hydrophilic polymer, number-average molecular weight technology, applied in the polymer field, can solve the problems of cumbersome introduction process and limited application scope, and achieve the effect of avoiding biological toxicity and hydrolysis stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
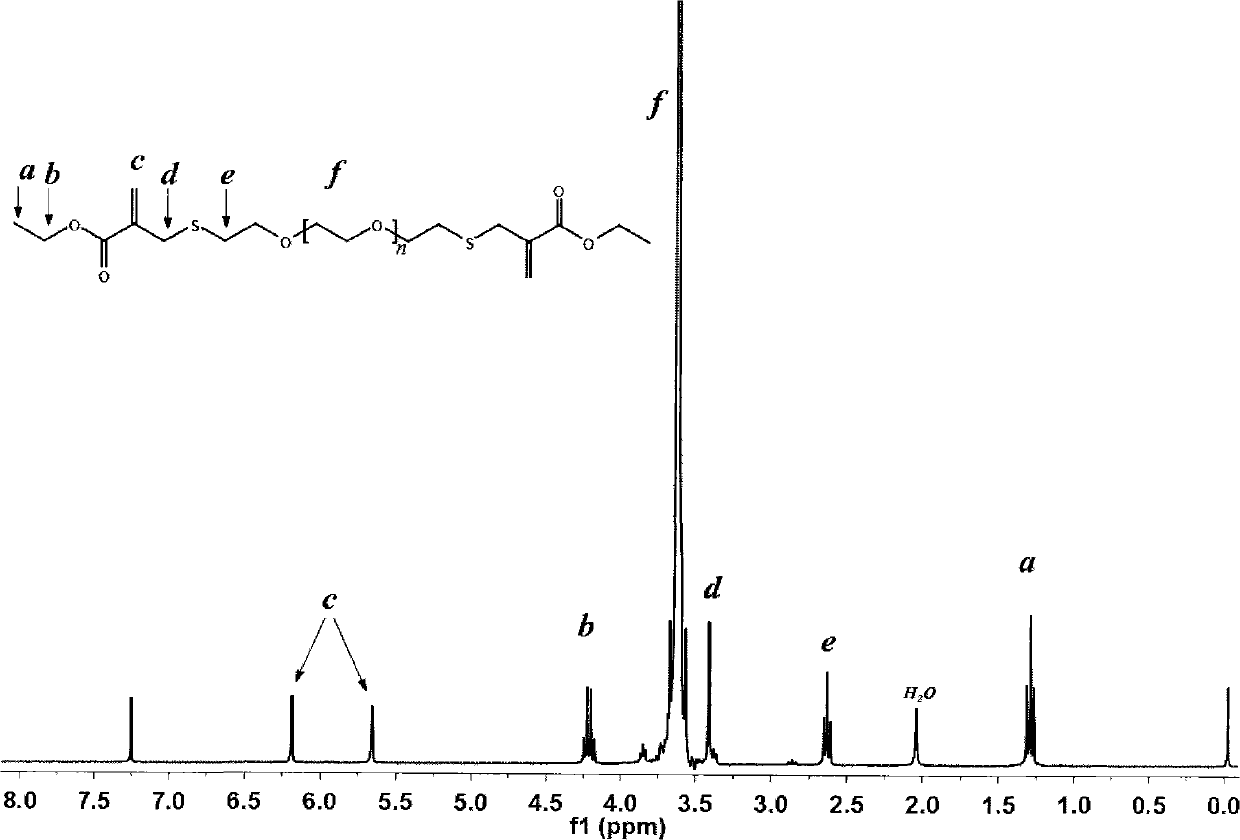
[0026] Example 1. Preparation of linear PEG that can undergo addition reaction with sulfhydryl groups and is bonded and hydrolytically stable
[0027] The compound represented by formula (II) was prepared by using PEG (number average molecular weight of about 2000, provided by Alfa-Aesar, product number B22181) with hydroxyl end group as raw material, wherein n is an integer greater than 30 and less than 60.
[0028]
[0029] Formula (II)
[0030] The specific preparation method is as follows:
[0031] Dissolve 20.0 g of hydroxyl-terminated PEG in 500 mL of dry dichloromethane, add 8.3 mL of triethylamine and 11.4 g of p-toluenesulfonyl chloride, and react at room temperature for 24 hours. Reflux for 4 hours, and then mix the obtained product with 2M ammonia in methanol and stir for 4 hours under the protection of nitrogen to obtain mercapto-terminated PEG.
[0032] Dissolve 9.0 g of ethyl hydroxymethacrylate (provided by TCI, product number H0916) in 75 mL of anhydrous e...
Embodiment 2
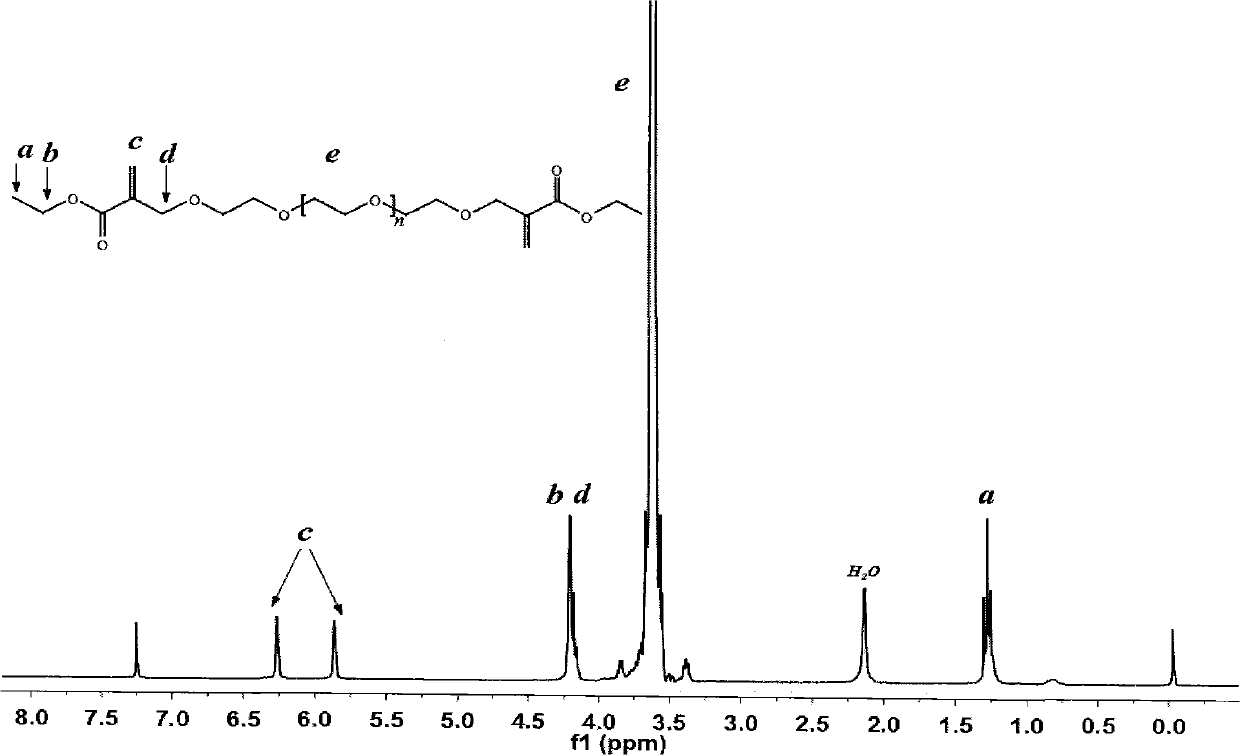
[0035] Example 2. Preparation of linear PEG that can undergo addition reaction with sulfhydryl groups and is stable in bond hydrolysis
[0036] The compound represented by formula (III) was prepared by using PEG with hydroxyl end group (the number average molecular weight is about 2000, provided by Alfa-Aesar, product number B22181) as raw material, wherein n is an integer greater than 30 and less than 60.
[0037]
[0038] Formula (III)
[0039] Ethyl bromomethacrylate can be obtained by the method in Example 1.
[0040] Dissolve 20.0 g of hydroxyl-terminated PEG in 300 mL of dichloromethane, add 2.4 g of sodium hydride, and stir for 1 hour. 8 mL of ethyl bromomethacrylate was added dropwise in an ice bath, and reacted at room temperature for 24 hours to obtain the product (compound represented by formula III).
[0041] The structure of the resulting compound can be obtained from figure 2 The H NMR spectrum was confirmed.
Embodiment 3
[0042] Example 3, hydrolytically stable in situ cross-linked hydrogel
[0043] The compound of formula II prepared in Example 1 and four-arm terminal mercapto PEG (number average molecular weight is about 10,000, provided by JenChem, product number 4ARM-SH-10K) were respectively dissolved in phosphate buffer at pH = 7.4 with a concentration of 0.1 mol / L The mass concentration of the two solutions is 0.1g / mL, and the two solutions are mixed at a volume ratio of 2:3, and the gel is formed within 7 hours after mixing evenly.
[0044] Or the compound of formula II prepared in Example 1 and the four-arm terminal mercapto PEG were respectively dissolved in phosphate buffer solution with a concentration of 0.1 mol / L at pH=8.0, and the mass concentration of both solutions was 0.1 g / mL. The two solutions were mixed at a volume ratio of 2:3, and gelled within 1 hour after mixing evenly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com