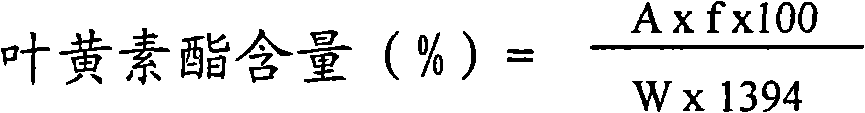Method for purifying lutein esters
A technology for lutein esters and esters, which is applied in the field of extracting high-purity lutein esters, can solve problems such as low yield, influence on product biological activity, and failure to meet high content requirements, and achieve the effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 101g marigold ointment (total lutein ester content is 29.3%) and mix with 404ml food grade ethyl acetate, dissolve by heating at 50℃, keep stirring for 3 hours, then hot filter the solution at 70℃ to remove insoluble matter , Slowly add 505ml of food grade ethanol, stir for 2.5 hours, let stand at 5℃ for 16 hours, filter the precipitated solid crystals, and finally wash the crystals with 300ml of n-butanol. The solid crystals were dried in a vacuum drying oven at a drying temperature of 40°C and a vacuum of 0.075 MPa, and finally 26.0 g of dried solid matter was obtained. The content was 78.6% by UV detection. The yield of total lutein esters was 69.1%. .
Embodiment 2
[0029] Weigh 107g marigold ointment (total lutein ester content is 29.3%) and mix with 428ml food grade ethyl acetate, heat to dissolve at 50℃, keep stirring for 3 hours, then hot filter the solution at 70℃ to remove insoluble matter , Slowly add 535ml of food grade ethanol, stir for 2.5 hours, let stand at 5°C for 16 hours, filter the precipitated solid crystals, and finally wash the crystals with 300ml of n-propanol. The solid crystals were dried in a vacuum drying oven at a drying temperature of 40° C. and a vacuum of 0.075 MPa. Finally, 21.3 g of crystals were obtained, the lutein ester content was 74.2%, and the total lutein yield was 50.4%.
Embodiment 3
[0031] Weigh 103g marigold ointment (total lutein ester content is 29.3%) and mix with 412ml food grade ethyl acetate, dissolve by heating at 50℃, keep stirring for 3 hours, then hot filter the solution at 70℃ to remove insoluble matter , Slowly add 515ml of food grade ethanol, stir for 2.5 hours, and then stand at 5°C for 16 hours, filter the precipitated solid crystals, and finally wash the crystals with 300ml of ethanol. The solid crystals were dried in a vacuum drying oven at a drying temperature of 40° C. and dried under a vacuum of 0.075 MPa. Finally, 19.3 g of crystals were obtained, the lutein ester content was 71.7%, and the total lutein yield was 45.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com