Amphiphilic copolymer brush with pH responsiveness, preparation method thereof and use thereof
An amphiphilic copolymer and responsive technology, which is applied in the field of medical polymer materials, can solve the problem that the cycle time and stability of the drug-loaded system cannot be guaranteed, the controlled release cannot be effectively realized, and the response is not accurate and sensitive enough and other problems, to achieve the effect of excellent product quality, increased density, and improved controlled release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
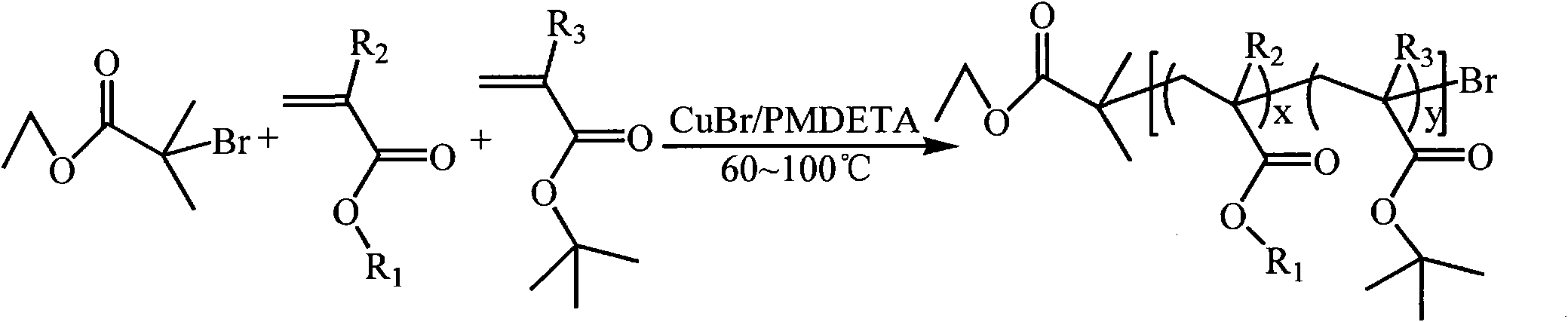
[0071] (1) Synthesis of P(MMA-co-tBMA)-Br macromolecular initiator (A:B=50:50, A represents the hydrophobic group MMA, B represents the pH-responsive group tBMA, the ratio is the mass percentage, the same below ). Take a 50ml dry reaction flask and put it into a stirrer. Weigh CuBr (143.5 mg) into a reaction flask, then seal it with a reverse rubber stopper, and vacuum-purge with argon three times. Monomer MMA (3181 μl), tBMA (5072 μl), and ligand PMDETA (209 μl) were sequentially added to the reaction flask by syringe, and the catalyst complex was formed by stirring for 10 minutes. After three cycles of freezing-pumping-heating with liquid nitrogen, the mixture was transferred to a 90°C oil bath under argon protection and stirred for 15 minutes, and then the initiator EBriB (147 μl) was rapidly added dropwise with a syringe. React in a 90°C oil bath for 30 minutes. After the reaction was completed, it was cooled to room temperature, 20 ml of tetrahydrofuran was added and s...
Embodiment 2
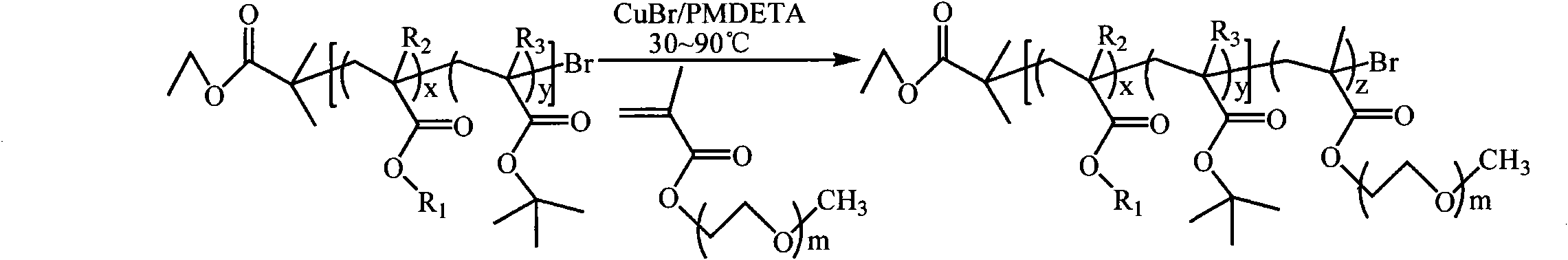
[0075] (1) Synthesis of P(BA-co-tBMA)-Br macroinitiator (A:B=40:60). Take a 50ml dry reaction flask and put it into a stirrer. Weigh CuBr (143.5 mg) into a reaction flask, then seal it with a reverse rubber stopper, and vacuum-purge with argon three times. Monomer BA (2237 μl) and tBMA (5072 μl), and ligand PMDETA (209 μl) were added to the vial sequentially by syringe and stirred for 10 minutes to allow the formation of the catalyst complex. After three cycles of freezing-pumping-heating with liquid nitrogen, the mixture was transferred to a 90°C oil bath under argon protection and stirred for 15 minutes, and then the initiator EBriB (147 μl) was rapidly added dropwise with a syringe. React in a 90°C oil bath for 30 minutes. After the reaction was completed, it was cooled to room temperature, 20 ml of tetrahydrofuran was added and stirred to dissolve, and then the catalyst was removed by filtration through a neutral alumina column (using tetrahydrofuran as the eluent). The...
Embodiment 3
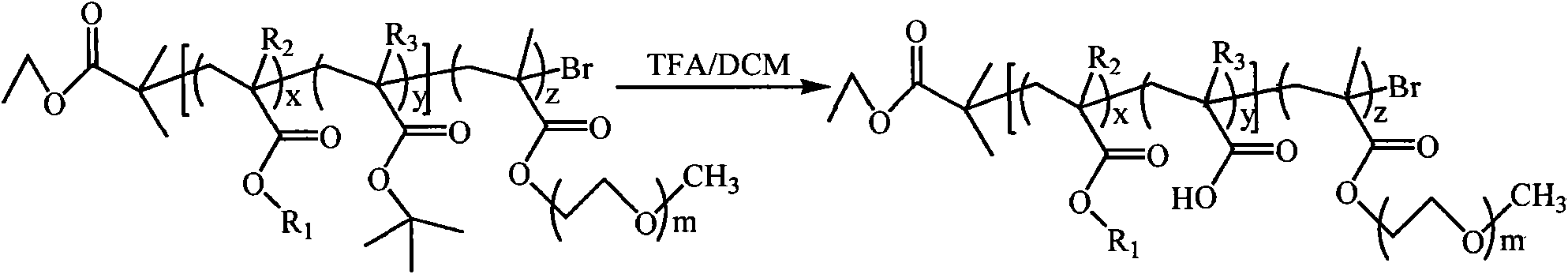
[0079] (1) Synthesis of P(BMA-co-tBMA)-Br macroinitiator (A:B=60:40). Take a 50ml dry reaction flask and put it into a stirrer. Weigh CuBr (143.5 mg) into a reaction flask, seal it with a rubber stopper, and evacuate-argon for three times. Monomer BMA (6710 μl) and tBMA (6760 μl), and ligand PMDETA (209 μl) were added to the vial sequentially by syringe and stirred for 10 minutes to allow the formation of the catalyst complex. After three cycles of freezing-pumping-heating with liquid nitrogen, the mixture was transferred to a 90°C oil bath under argon protection and stirred for 15 minutes, and then the initiator EBriB (147 μl) was rapidly added dropwise with a syringe. React in a 90°C oil bath for 45 minutes. After the reaction was completed, it was cooled to room temperature, 50 ml of tetrahydrofuran was added and stirred to dissolve, and then the catalyst was removed by filtration through a neutral alumina column (using tetrahydrofuran as the eluent). The resulting solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Critical micelle concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com