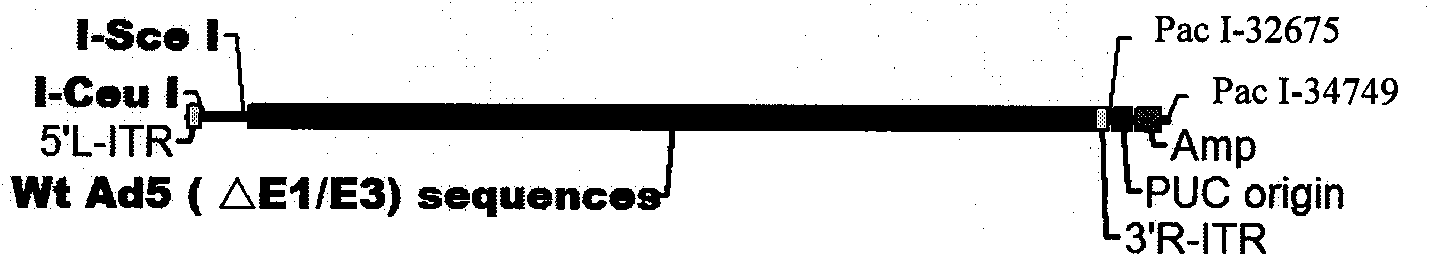Recombinant adenovirus carrier and application thereof
A recombinant adenovirus, vector plasmid technology, applied in the direction of virus/bacteriophage, recombinant DNA technology, introduction of foreign genetic material using vectors, etc., can solve the problems of low survival rate of malignant tumor patients and poor prognosis of solid tumors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1, the preparation of Ad-DENND2D recombinant adenovirus
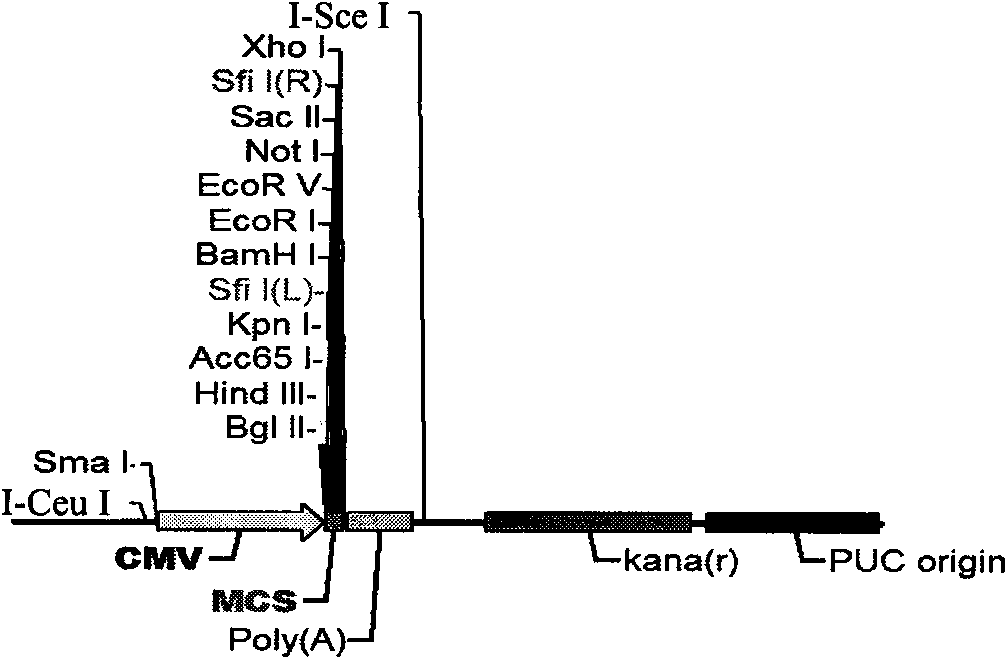
[0053] pShuttle-CMV shuttle vector (see figure 1 ): Nosy Genetics.
[0054] pAdxsi adenoviral backbone vector (see figure 2 ): Nosy Genetics.
[0055] 1. Construction of pAdxsi-DENND2D recombinant plasmid
[0056] 1. Construction of DENND2D recombinant shuttle vector
[0057] The primers of the DENND2D gene with the SfiI restriction endonuclease site were designed as follows:
[0058] DENND2D Sfi I up: 5'-AAAAAAGGCCGCTGCGGCCCACTCCAGGGGCCATGGATG-3';
[0059] DENND2D Sfi I dw: 5'-AAAAAAGGCCTGTTTGGCCGTCATTCTTATTCACCACAGCTC-3'.
[0060] ①Use the above primers to obtain the full-length coding region of the target DENND2D gene by PCR from the human lung tissue cDNA library (purchased from Clontech Company). 3-4 hours. A 1.4kb fragment was recovered.
[0061] ② The pShuttle-CMV shuttle vector was digested with restriction endonuclease SfiI at 37°C for 3-4 hours, and then the vector was dephosphoryla...
Embodiment 2
[0105] Example 2, Induction of apoptosis in vitro by Ad-DENND2D recombinant adenovirus
[0106] Infect the human lung cancer cell line H1299 cells with Ad-DENND2D (or Adv) according to the dose of moi 100 or moi 200 respectively, collect the cells after 48 hours for Annexin V-FITC and PI double staining, and detect cell apoptosis by flow cytometry Happened. The experiment was repeated three times, and the results were averaged.
[0107] see results Figure 5 (The figure shows the results of one of the experiments). After infection with a dose of moi100, the apoptosis rate of Ad-DENND2D group was 17.09±3.22%, which was significantly higher than that of Adv group (11.36±1.25) (P<0.05). After infection with a dose of moi200, the apoptosis rate of the Ad-DENND2D group was 21.76±1.99%, which was significantly higher than that of the Adv group (14.06) (P<0.05). For cells infected with Ad-DENND2D, the apoptosis rate of the moi200 dose group was higher than that of the moi100 dose...
Embodiment 3
[0108] Example 3, In vivo anti-tumor effect of Ad-DENND2D recombinant adenovirus
[0109] 1. Select 24 female BALB / c nude mice (purchased from Victoria Lihua Company) aged 3 to 4 weeks; collect H1299 cells in the logarithmic growth phase (purchased from ATCC), and adjust the cell concentration to 2.5×10 7 / ml.
[0110] 2. Take 200 μl of cell suspension and inoculate it subcutaneously in the right armpit of nude mice. After about 10 days, when the tumor grows to a size of 5×5 mm, divide them into four groups, with 6 mice in each group, and perform the following treatments:
[0111] Normal saline injection group (NS): injected with normal saline, once every three days at multiple points in the tumor, 50 μl each time, three times in total.
[0112] Adv virus group (Adv): adjust the Adv virus titer to be 2×10 10 pfu / ml, intratumoral multi-point injection once every three days, 50 μl each time, a total of three times, the cumulative virus injection volume was 3×10 9 pfu.
[011...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com