Method for preparing sandaler
The technology of a kind of sandalwood ether and preparation steps is applied to the green process route of synthesizing sandalwood ether, and the preparation field of sandalwood ether can solve the problems of poor hydrogenation selectivity, high equipment requirements and high catalyst price, and achieves improvement of synthesis process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
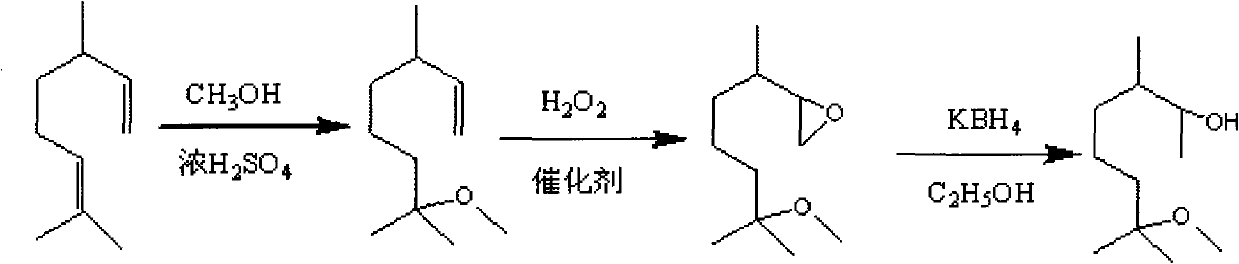
[0028] (1), Synthesis of 3,7-dimethyl-7-methoxyoctene
[0029] At room temperature, 405ml (10.0mol) of methanol was added in a 1000ml three-necked round-bottomed flask, and 182ml of dihydromyrcene (industrial product, content about 85%, 1.0mol) was added dropwise under stirring, and 22ml of concentrated sulfuric acid (content greater than 1.0mol) was slowly added dropwise. 98%, 0.4mol), after feeding, the temperature was raised to 65°C and refluxed for 5 hours, then cooled to room temperature, washed with water, and the organic layer was separated and washed with 5% NaHCO 3 The aqueous solution was washed to neutrality, and the obtained organic layer was subjected to vacuum distillation to reclaim 22.8 g of dihydromyrcene as a raw material (recyclable), and collected product fraction 3,7-dimethyl-7-methoxyoctene 115.7 g g (gas phase content is 96.9%), productive rate is 81.3%;
[0030] (2), synthesis of 3,7-dimethyl-7-methoxy-1,2-epoxyoctane
[0031] Catalysts for epoxidatio...
Embodiment 2
[0036] (1), Synthesis of 3,7-dimethyl-7-methoxyoctene
[0037] At room temperature, 324ml (8.0mol) of methanol was added in a 1000ml three-necked round-bottomed flask, and 182ml of dihydromyrcene (industrial product, content about 85%, 1.0mol) was added dropwise under stirring, and 22ml of concentrated sulfuric acid (content greater than 1.0mol) was slowly added dropwise. 98%, 0.4mol), after feeding, the temperature was raised to 65°C and refluxed for 5 hours, then cooled to room temperature, washed with water, and the organic layer was separated and washed with 5% NaHCO 3 The aqueous solution was washed to neutrality, and the obtained organic layer was subjected to vacuum rectification to recover 47.6 g of dihydromyrcene as a raw material (recyclable), and 92.0 g of product fraction 3,7-dimethyl-7-methoxyoctene was collected. g (gas phase content is 96.5%), productive rate is 82.4%;
[0038] (2), synthesis of 3,7-dimethyl-7-methoxy-1,2-epoxyoctane
[0039] Catalysts for epo...
Embodiment 3
[0044] (1), Synthesis of 3,7-dimethyl-7-methoxyoctene
[0045] At room temperature, 486ml (12.0mol) of methanol was added in a 1000ml three-neck round-bottomed flask, and 182ml of dihydromyrcene (industrial product, content about 85%, 1.0mol) was added dropwise under stirring, and 22ml of concentrated sulfuric acid (content greater than 1.0mol) was slowly added dropwise. 98%, 0.4mol), after feeding, the temperature was raised to 65°C and refluxed for 5 hours, then cooled to room temperature, washed with water, and the organic layer was separated and washed with 5% NaHCO 3 The aqueous solution was washed to neutrality, and the obtained organic layer was subjected to vacuum distillation to recover 22.3 g of dihydromyrcene (recyclable) as the raw material, and 119.1 g of product fraction 3,7-dimethyl-7-methoxyoctene was collected. g (gas phase content is 96.7%), productive rate is 83.4%;
[0046] (2), synthesis of 3,7-dimethyl-7-methoxy-1,2-epoxyoctane
[0047] Catalysts for ep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com