Orally disintegrating tablet compositions of temazepam
A technology of orally disintegrating tablet and composition, which is applied in the direction of drug combination, drug delivery, medical preparations containing active ingredients, etc., and can solve problems such as undesired taste or mouthfeel characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0096] Example 1A - Rapidly Dispersing Microparticles
[0097] Prepared by mixing D-Mannitol (having an average particle size of about 15 μm) with Crospovidone XL-10 in a ratio of about 95 / 5 in a high shear granulator using purified water as the granulation fluid rapidly dispersing microparticles. The resulting rapidly dispersing microparticles were dried by dispersing the granulated mixture in the trays of a heated convection oven. The average particle size of the dried fast-dispersing microparticles is less than about 400 μm.
example 1
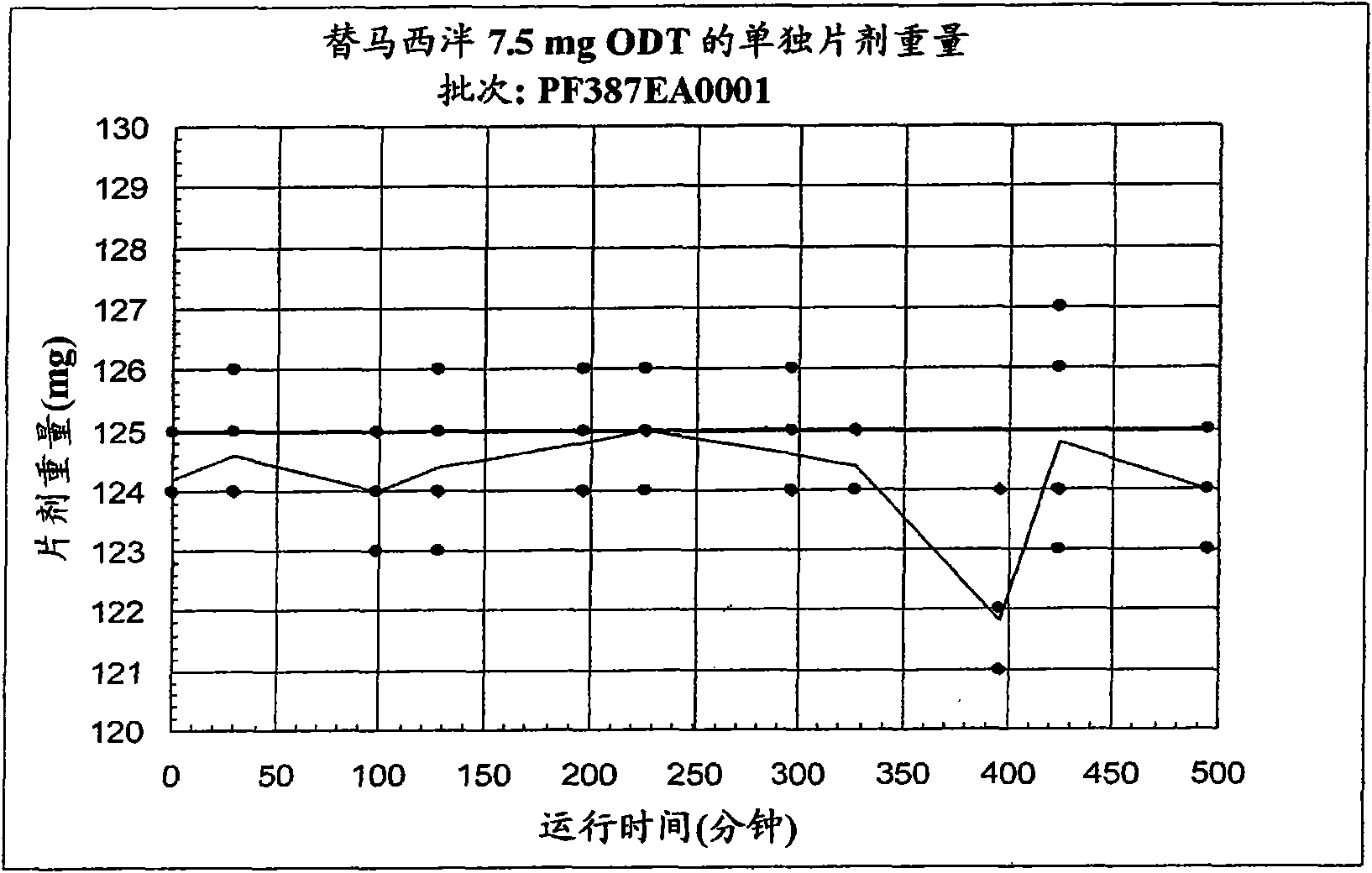
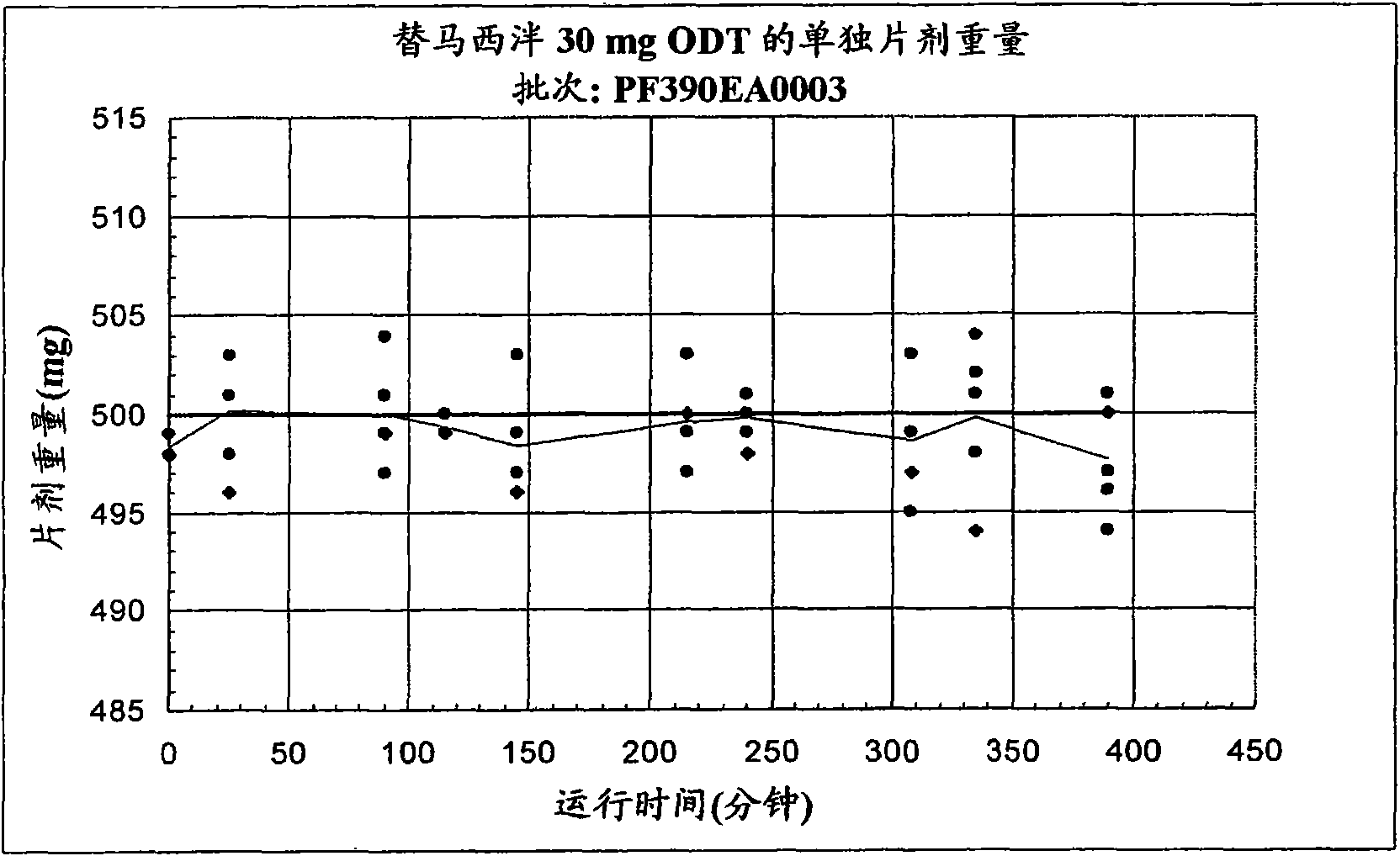
[0098] Example 1B - 30 mg temazepam ODT
[0099] Sucralose, cherry or mint flavor, crospovidone XL-10, and microcrystalline cellulose were pre-blended, then blended with crystalline temazepam, and compressed into 30 mg tablets using a Hata tablet press machine and 11mm, round, standard, concave tooling, a vacuum transfer system, tablet dust collector, a metal detector, and a Matsui ExLub system. The ExLub system sprays a lubricant, such as magnesium stearate, at a preselected rate into the die cavity and onto the punch face, and then vacuums out excess lubricant prior to pressing. The punch and die surfaces were externally lubricated with magnesium stearate so that only traces of the lubricant were present in the tablet. The tableting apparatus was adjusted by varying the compression force from about 8 kN to about 16 kN to provide tablets with a friability of less than 1% and a hardness of about 30N. The relative standard deviation (RSD) of tablet weight (target weight: 500 ...
example 2
[0101] Example 2A - Temazepam Microparticles
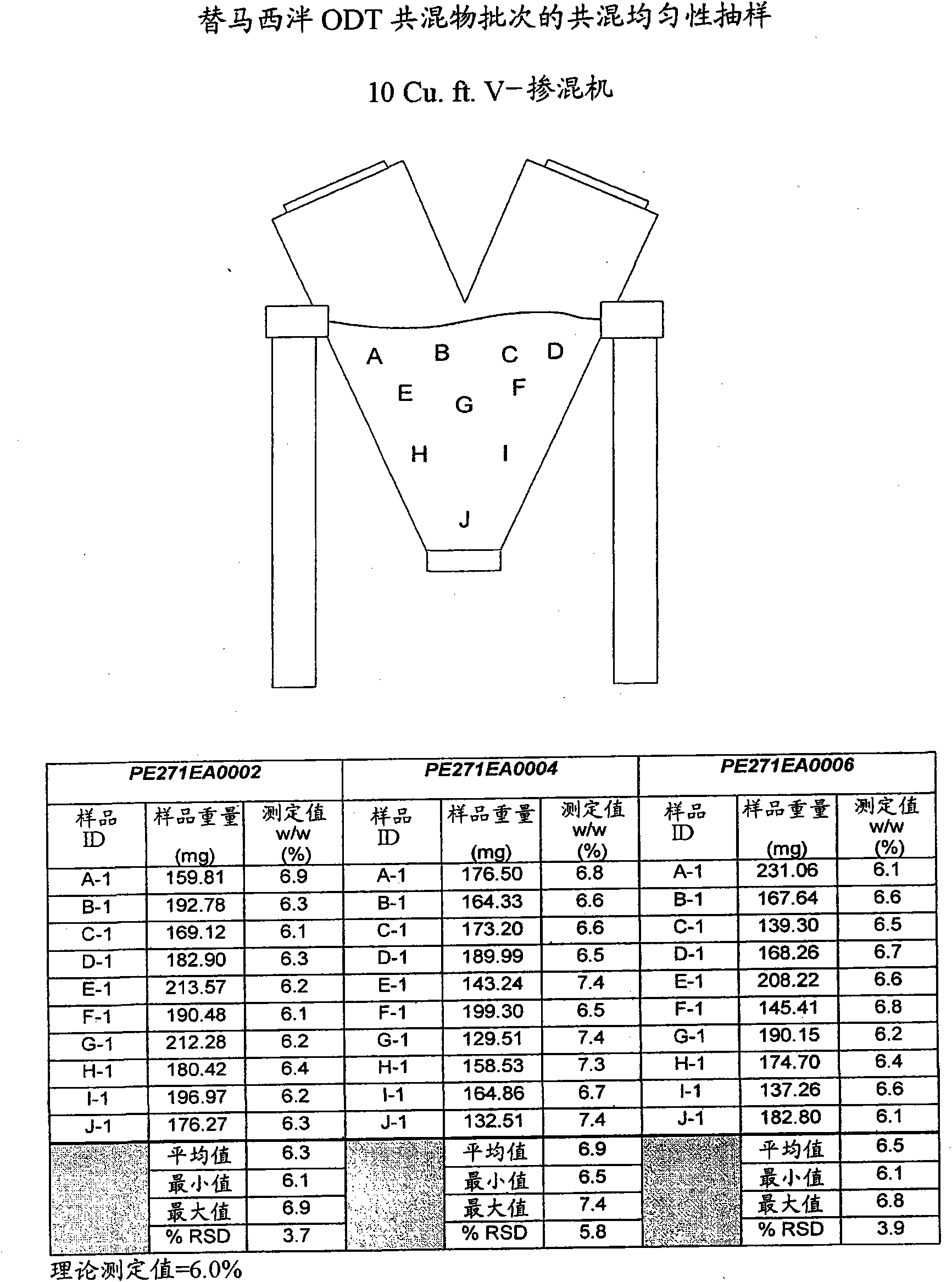
[0102] The mixture was granulated by charging temazepam, mannitol, and crospovidone into a Glatt GPCG 5 fluid bed granulator, and using purified water as a granulation fluid (batch size 6 kg). Temazepam microparticles were prepared. Batches were made with drug concentrations of approximately 6.3% w / w, 15.0% w / w and 30.0% w / w to evaluate the effect of increasing the concentration of temazepam on the quality of the pellets produced. Mannitol sticking to the sides of the fluid bed processor was observed during the manufacture of these batches, resulting in a bimodal particle size distribution with significant fines fraction fines, which in turn caused unstable flow characteristics.
[0103] Example 2B - Temazepam ODT (7.5, 15, 22.5 and 30 mg Temazepam doses)
[0104] Different ODT compositions were evaluated to determine the "firmness" of the formulation (e.g. the effect of the following on ODT properties such as hardness, friabil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com