Device and method for preparing lithium carbonate from brine by utilizing solar energy
A technology of lithium carbonate and solar energy, applied in the direction of lithium carbonate;/acid carbonate, energy input, etc., can solve the problems of limited promotion of lithium extraction from brine and comprehensive utilization technology, limited production efficiency, etc., and achieve production efficiency High, obvious energy-saving advantages, and the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Sulfate-type brine with a magnesium-lithium ratio of 60 is used as raw material, the composition of the raw brine is shown in Table 1, and the density is 1.11g / cm 3 .
[0025] Table 1 Composition of raw brine (g / L)
[0026] Na +
K +
Li +
Ca 2+
Mg 2+
Cl -
SO 4 2-
15.778
2.376
0.047
0.087
2.840
23.749
5.205
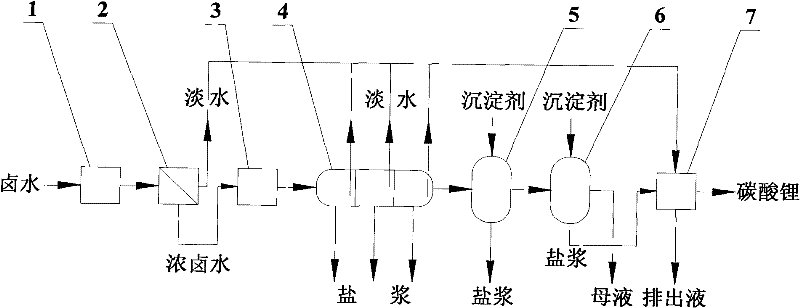
[0027] The working process and operation method of the device for producing lithium carbonate from brine using solar energy are as follows.
[0028] a) feeding the brine into the first solar heat collector, and heating the brine to 70° C. with solar heat;
[0029] b) The heated brine enters the membrane still, and the membrane still uses hydrophobic polypropylene microporous membrane elements for direct contact operation. The brine is separated into fresh water and concentrated brine with higher salt content through the membrane distillation process. The fresh wate...
Embodiment 2
[0037] The raw material brine composition and device are the same as in Example 1, and the work flow and operation method are as follows.
[0038] a) feeding the brine into the first solar heat collector, and heating the brine to 60° C. with solar heat;
[0039] b) The heated brine enters the membrane still, and the membrane still adopts hydrophobic polypropylene microporous membrane elements and operates in vacuum mode. The brine is separated into fresh water and concentrated brine with higher salt content through the membrane distillation process. The fresh water enters the salt scrubber, and the concentrated brine whose temperature drops to 40°C is sent to the second solar collector; the concentration ratio is controlled during the membrane distillation process. Around 1.6, no solid-phase salt was precipitated during the whole process;
[0040] c) In the second solar heat collector, after the concentrated brine is heated to 60°C, it is then sent to the triple-effect evapor...
Embodiment 3
[0047] Raw material brine composition, device and work flow are identical with embodiment 1, and difference is that the first crystallizer adds Na 2 CO 3 Before the precipitant, add Na to the first crystallizer in sequence 2 SO 4 and Ca(OH) 2 , forming a MgCO containing 3 , Mg(OH) 2 and CaSO 4 2H 2 O's salt slurry.
[0048] Li obtained in this example 2 CO 3 In the product, Li 2 CO 3 The mass content reached 75.96%, and the Li yield in the whole process reached 89.63%, which took 55 hours (including crystallization aging time).
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com