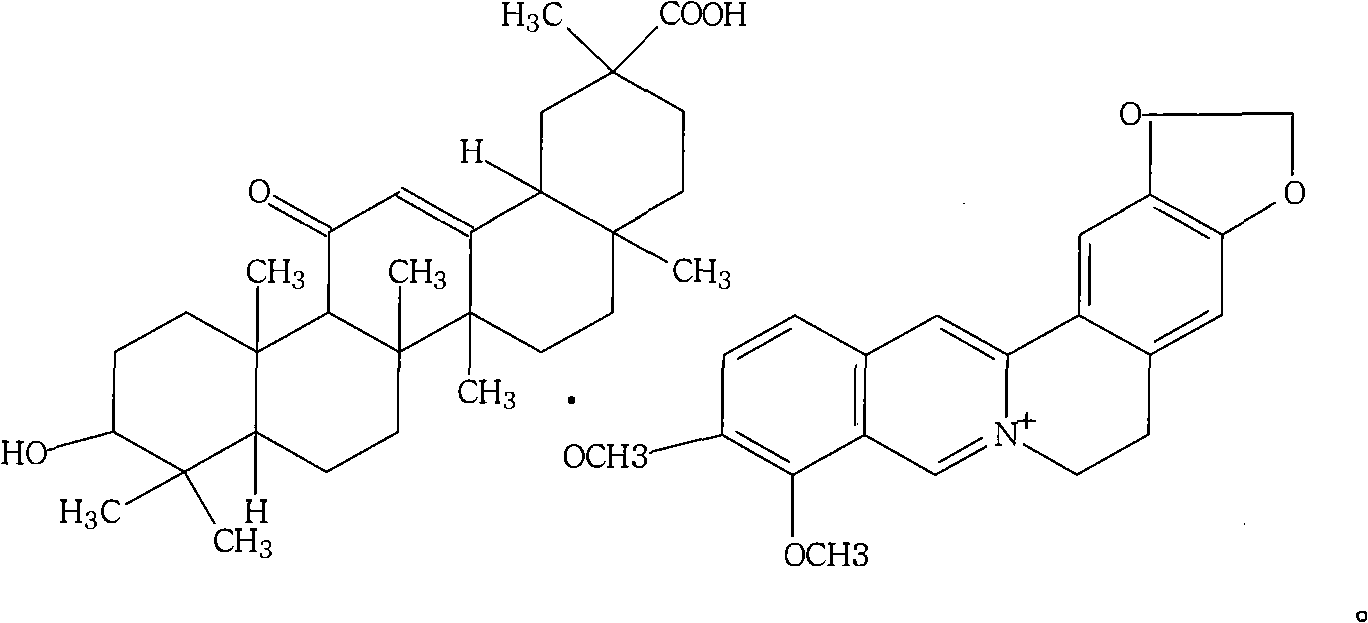
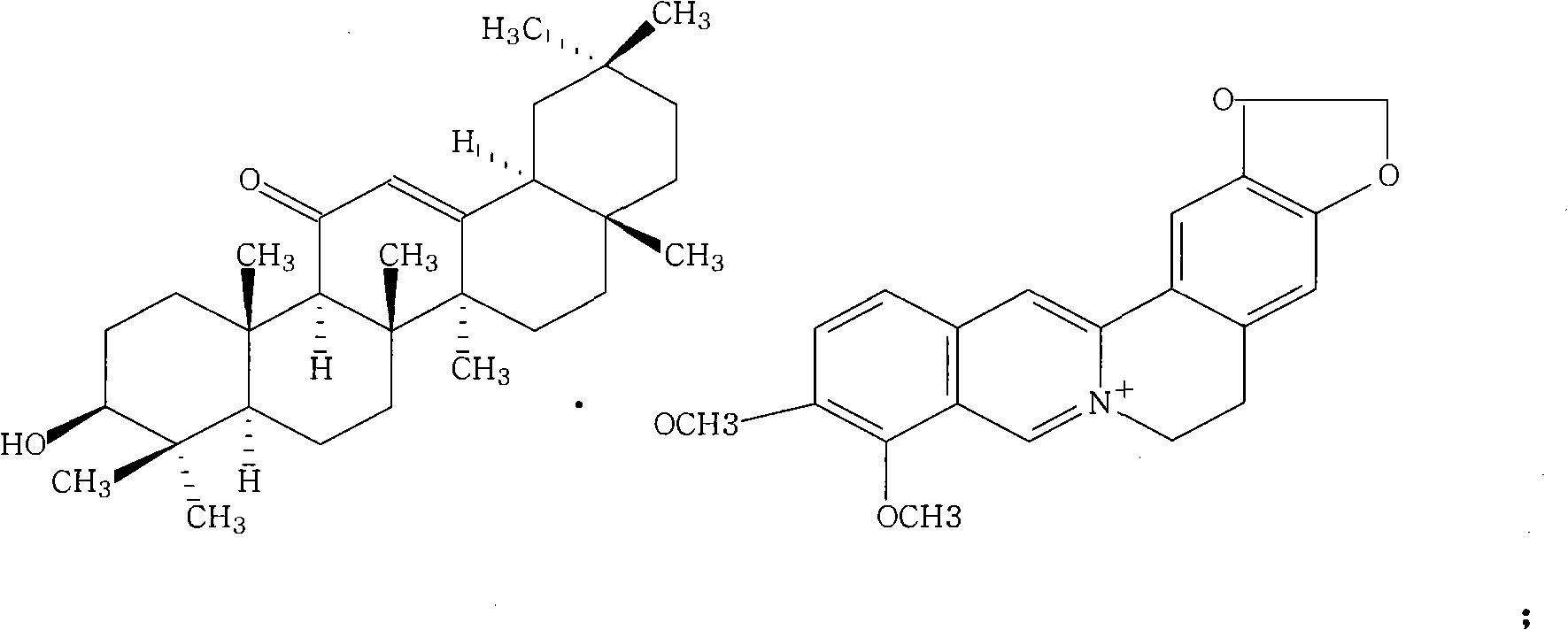
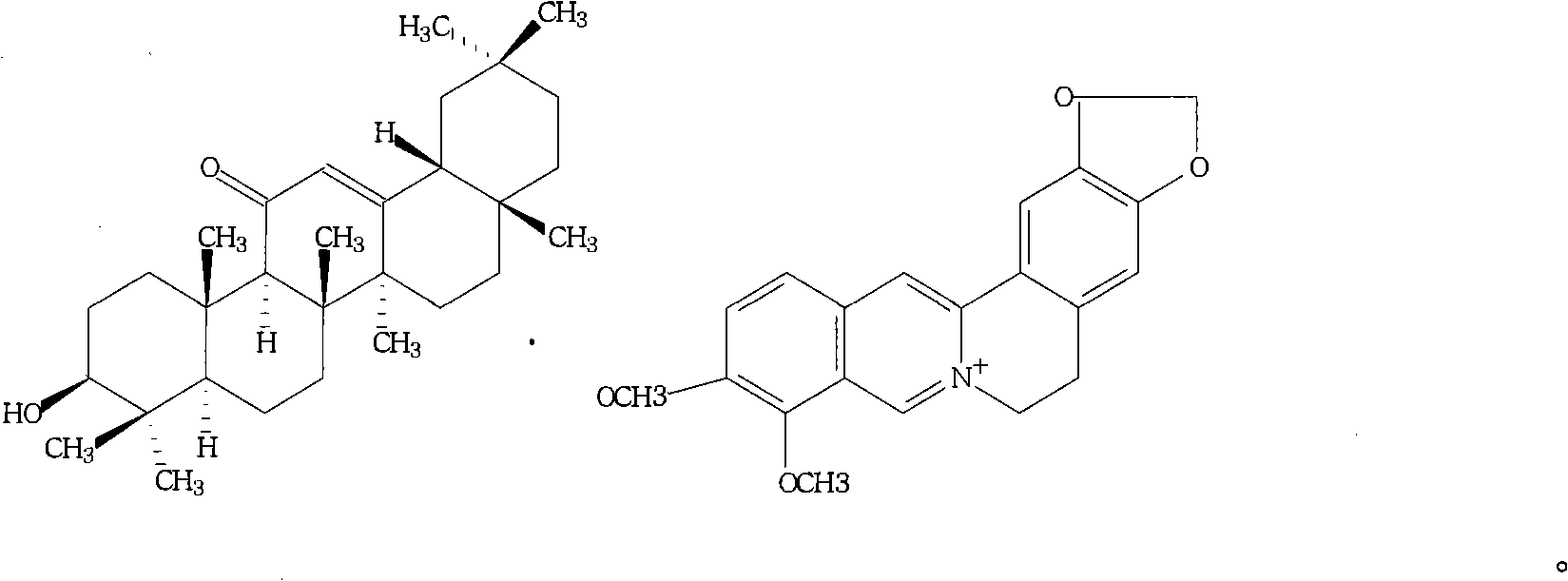
Berberine glycyrrhetinic acid enantiomeric salts and preparation method and application thereof
A technology of glycyrrhetinic acid and berberine, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, digestive system, etc., can solve the problems of berberine's clinical curative effect and insufficient therapeutic range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The 5,6-dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolane[5,6-a]quinazine·18β,3β-hydroxy -11-Oxooleanane-12-ene-30-carboxylate: Its preparation method is:
[0026] Take 84g of berberine and dissolve with 80% ethanol, and dissolve 117.5g of 18β-glycyrrhetinic acid with 80% ethanol, add the above solution and mix and stir for 2 hours, remove the solvent under reduced pressure, grind the residue with a small amount of ethyl acetate, precipitate, filter, reduce Press and dry to obtain 104.3 g of light yellow powder with a yield of 51.7%.
[0027] mp: 316~321℃
[0028] [C] D20+170.0° (c=1, chloroform)
[0029]
Embodiment 2
[0031] The 5,6-dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolane[5,6-a]quinazine·18a,3β-hydroxy The preparation method of -11-oxooleanane-12-ene-30-formate is:
[0032] Take 84g of berberine and dissolve with 80% ethanol, dissolve 117.5g of 18a-glycyrrhetinic acid with absolute ethanol, add the above solution and mix and stir for 2 hours, remove the solvent under reduced pressure, grind the residue with a small amount of ethyl acetate, precipitate, filter, reduce Press and dry to obtain 112.6 g of light yellow powder with a yield of 55.9%.
[0033] mp: 346~351℃
[0034] [C] D20+98° (c=1, chloroform)
[0035]
[0036]
Embodiment 3
[0038] This embodiment describes the effect of the compounds of the present invention on the blood sugar of alloxan diabetic mice:
[0039]Alloxan was used to create a hyperglycemia model in mice, and the mice with blood glucose>11mmol / l were randomly divided into model control group, 18β-glycyrrhetinic acid berberine salt, 18a-glycyrrhetinic acid berberine salt, positive control group, Give normal saline, 18β-glycyrrhetinic acid berberine salt, 18a-glycyrrhetinic acid berberine salt, and berberine, once a day, for 14 consecutive days, fasting for 12 hours after the last administration, without water, and testing for fasting Blood sugar, observe changes in blood sugar before and after medication.
[0040] Table 1:
[0041] Blood glucose before and after alloxan administration in mice
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com