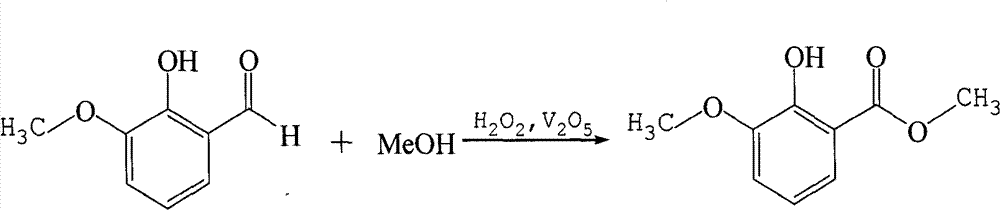Preparation method for 2-hydroxy-3-methoxy-methyl benzoate
A technology of methyl methoxybenzoate and methoxybenzaldehyde, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as long synthetic routes, achieve the effects of simplifying operations, reducing by-products, and increasing overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1, 2-hydroxyl-3-methoxybenzaldehyde is reacted with methanol, vanadium pentoxide and hydrogen peroxide, the reaction of 2-hydroxyl-3-methoxybenzaldehyde with methanol, vanadium pentoxide, hydrogen peroxide The molar ratio is 1:50:0.3:1.2, the reaction temperature is 5°C, and the reaction time is 8 hours.
[0018] 2. After the above steps are completed, filter, distill the filtrate under reduced pressure and condense to recover methanol, extract with diethyl ether, distill under reduced pressure and recover diethyl ether to obtain a crude product.
[0019] 3. The crude product was subjected to silica gel column chromatography to obtain methyl 2-hydroxy-3-methoxybenzoate.
[0020] The methanol recovered by distillation in step 2 is used for the reaction of step 1, the diethyl ether recovered by distillation can be reused in step 2, and the filter residue after filtering in step 2 is collected and processed to avoid environmental pollution.
Embodiment 2
[0022] 1, 2-hydroxyl-3-methoxybenzaldehyde is reacted with methanol, vanadium pentoxide and hydrogen peroxide, the reaction of 2-hydroxyl-3-methoxybenzaldehyde with methanol, vanadium pentoxide, hydrogen peroxide The molar ratio is 1:200:0.5:1.6, the reaction temperature is 25° C., and the reaction time is 4 hours.
[0023] 2. After the above steps are completed, filter, distill the filtrate under reduced pressure and condense to recover methanol, extract with diethyl ether, distill under reduced pressure and recover diethyl ether to obtain a crude product.
[0024] 3. The crude product was subjected to silica gel column chromatography to obtain methyl 2-hydroxy-3-methoxybenzoate.
[0025] The methanol recovered by distillation in step 2 is used for the reaction of step 1, the diethyl ether recovered by distillation can be reused in step 2, and the filter residue after filtering in step 2 is collected and processed to avoid environmental pollution.
Embodiment 3
[0027] 1, 2-hydroxyl-3-methoxybenzaldehyde is reacted with methanol, vanadium pentoxide and hydrogen peroxide, the reaction of 2-hydroxyl-3-methoxybenzaldehyde with methanol, vanadium pentoxide, hydrogen peroxide The molar ratio is 1:500:0.7:2, the reaction temperature is 50° C., and the reaction time is 1 hour.
[0028] 2. After the above steps are completed, filter, distill the filtrate under reduced pressure and condense to recover methanol, extract with diethyl ether, distill under reduced pressure and recover diethyl ether to obtain a crude product.
[0029] 3. The crude product was subjected to silica gel column chromatography to obtain methyl 2-hydroxy-3-methoxybenzoate.
[0030] The methanol recovered by distillation in step 2 is used for the reaction of step 1, the diethyl ether recovered by distillation can be reused in step 2, and the filter residue after filtering in step 2 is collected and processed to avoid environmental pollution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com