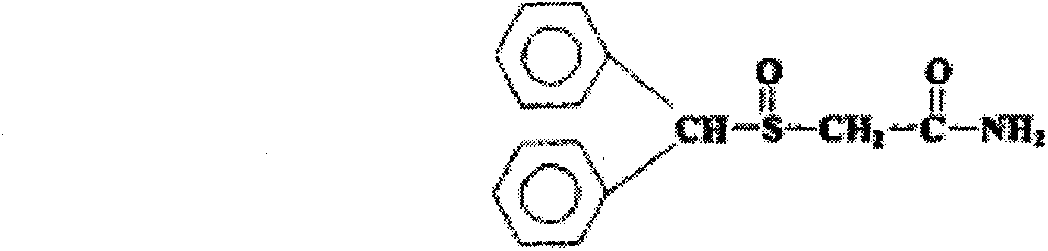
Modafinil dispersible tablet and preparation method thereof
A technology of dispersible tablets and weight ratio, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve the problems of dissolution and bioavailability that are difficult to achieve therapeutic effects. To achieve the effect of good short-term tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (Specification: 200mg / tablet)
[0033] formula:
[0034]
[0035] preparation:
[0036] (1) Pulverize modafinil through a 260 mesh sieve.
[0037] (2) Carboxymethyl starch sodium and microcrystalline cellulose are respectively passed through a 200-mesh sieve.
[0038] (3) The starch is passed through an 80-mesh sieve.
[0039] (4) Micropowder silica gel is passed through a 300-mesh sieve, and stearic acid is passed through a 200-mesh sieve.
[0040] (5) Modafinil, microcrystalline cellulose, starch and half of the formula amount of carboxymethyl starch sodium and starch are mixed uniformly according to the ratio of the recipe amount, and the soft material is made with 1% PVP aqueous solution, and the wet granules are made through a 20-mesh sieve. Ventilate and dry at 60°C (the moisture content of the dry particles is 2%), and granulate with a 20-mesh sieve;
[0041] (6) Micropowder silica gel, stearic acid and the remaining amount of sodium carboxymethyl starch ...
Embodiment 2
[0043] (Specification: 200mg / tablet)
[0044] formula:
[0045]
[0046]
[0047] preparation:
[0048] (1) Crushing the main ingredient through a 260-mesh sieve.
[0049] (2) Low-substituted hydroxypropyl cellulose and microcrystalline cellulose are respectively passed through a 200-mesh sieve.
[0050] (3) The starch is passed through an 80-mesh sieve.
[0051] (4) Micropowder silica gel is passed through a 300-mesh sieve, and stearic acid is passed through a 200-mesh sieve.
[0052] (5) Mix modafinil, microcrystalline cellulose, starch and half of the low-substituted hydroxypropyl cellulose and starch according to the proportion of the prescription, and use 1% PVP aqueous solution to make a soft material, and use a 20-mesh sieve to make a wet The granules are ventilated and dried at 60°C (the moisture content of the dry granules is 2%), and granulated with a 20-mesh sieve;
[0053] (6) Micropowder silica gel, stearic acid and the remaining amount of low-substitut...
Embodiment 3
[0055] (Specification: 200mg / tablet)
[0056] formula:
[0057]
[0058]
[0059] preparation:
[0060] (1) Crushing the main ingredient through a 260-mesh sieve.
[0061] (2) Carboxymethyl starch sodium, lactose, and mannitol were respectively passed through a 200-mesh sieve.
[0062] (3) Micropowder silica gel is passed through a 300-mesh sieve, and stearic acid is passed through a 200-mesh sieve.
[0063] (4) Mix modafinil, lactose, mannitol and sodium carboxymethyl starch with half of the formula quantity and starch according to the proportion of the prescription quantity, and use 1% sodium carboxymethylcellulose aqueous solution to make the soft material, and sieve it with 20 mesh Wet granules are ventilated and dried at 60°C (moisture content of dry granules is 2%), and granulated with a 20-mesh sieve;
[0064] (5) Micropowder silica gel, stearic acid and the remaining amount of sodium carboxymethyl starch are fully mixed, punched with a 9mm shallow arc, and pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com