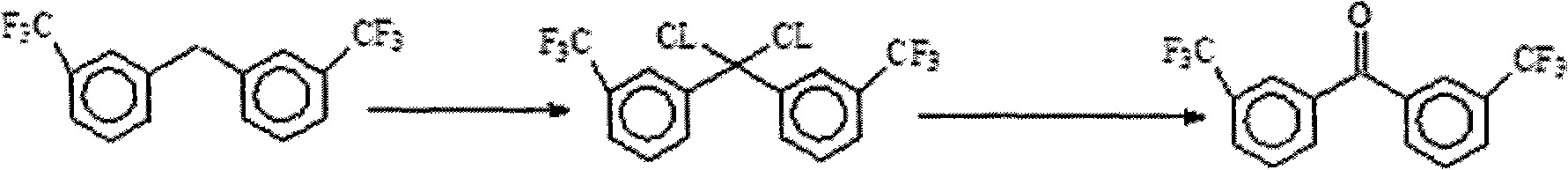
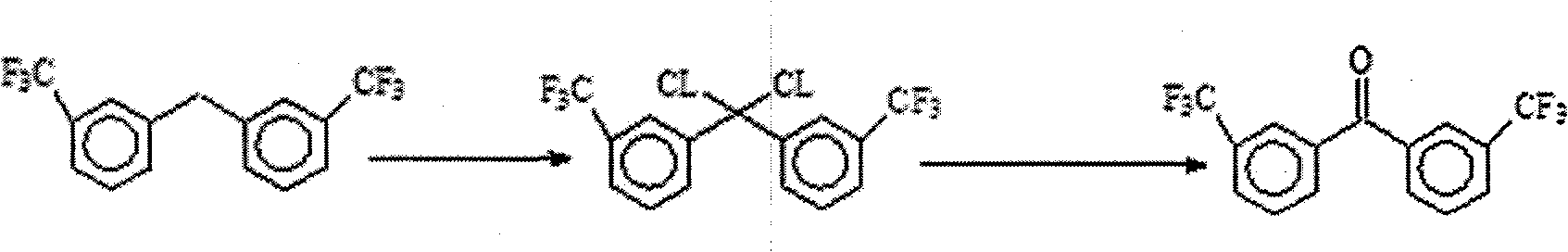Method for preparing 3,3'-bis (trifluoromethyl) benzophenone
A technology of trifluoromethyl and benzophenone, applied in 3 fields, can solve the problems of difficult industrialization of Grignard reaction, and achieve the effects of easy industrialization, low production cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Mix 10 grams of raw material 3,3'-bis(trifluoromethyl)benzenemethane, 100 grams of solvent chloroform and 0.2 gram of catalyst phosphorus pentachloride, then under the illumination of 100w incandescent light and reflux at 60°C, pass 7 grams in 20 hours Chlorine gas, the reaction process was followed by GC and NMR;
[0024] After the reaction is completed, cool, add water, extract with chloroform, separate layers, and after the oil layer is concentrated, the intermediate colorless oily substance 3,3'-bis(trifluoromethyl)phenyldichloromethane is obtained;
[0025] The intermediate 3,3`-bis(trifluoromethyl)phenyldichloromethane, 30 grams of water, 12 grams of acetonitrile and 3 grams of sodium hydroxide were mixed and refluxed for 4 hours, cooled and filtered to obtain the crude product, which was recrystallized with ethanol to obtain 7.8 g of the target product as white crystals, 3,3'-bis(trifluoromethyl)benzophenone, melting point: 102-104°C.
[0026] The yield of the a...
Embodiment 2
[0028] 10 grams of raw material 3,3'-bis(trifluoromethyl)benzene methane, 200 grams of solvent carbon tetrachloride and 0.3 gram of catalyst phosphorus pentachloride were mixed, then under the illumination of 100w incandescent lamp, 12 hours under reflux at 80 DEG C 9.5 grams of chlorine gas, the reaction process was followed by GC and NMR; the reaction was completed, cooled, added water, extracted with carbon tetrachloride, layered, and the oil layer was concentrated to obtain the intermediate colorless oil 3,3`-bis(trifluoromethyl ) phenyl dichloromethane.
[0029] The intermediate 3,3`-bis(trifluoromethyl)phenyldichloromethane mixed with 50 grams of water, 15 grams of acetonitrile and 5 grams of potassium hydroxide was refluxed for 4 hours, cooled and filtered to obtain the crude product, which was recrystallized with ethanol to obtain 8.9 g of the white crystalline target product 3,3'-bis(trifluoromethyl)benzophenone, melting point: 102-104 degrees. The yield of the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com