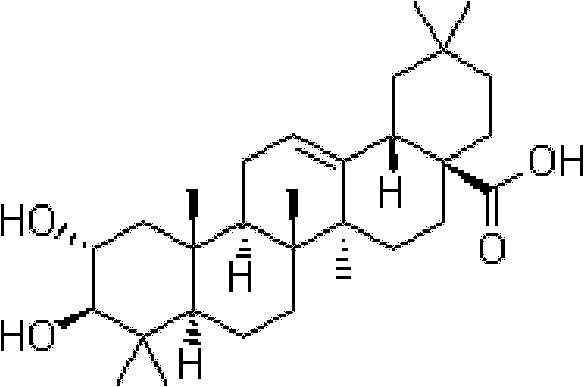
Method for synthesizing crataegolic acid
A technology of maslinic acid and oleanolic acid, applied in the direction of steroids, organic chemistry, etc., can solve problems such as complicated steps, extreme reaction conditions, and cumbersome process routes, and achieve easy control of reaction conditions, satisfactory reaction conditions, and excellent reaction environment friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
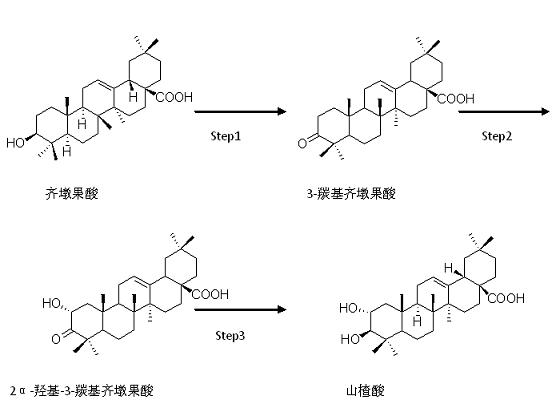
[0026] Step 1) in N 2 Under protection, 14.07g of oleanolic acid was dissolved in a mixed solvent of 300ml of acetone and 100ml of DMF in a 1000ml four-necked flask. 45ml of Jones reagent was slowly added dropwise at 0°C. After the addition, it was raised to room temperature and reacted for 1 hour; monitoring the completion of the reaction, Cool down to 0°C and slowly add 300ml isopropanol dropwise. After the dripping was completed, the reaction system was poured into ice water to precipitate solids, washed repeatedly with water, filtered with suction, and dried under vacuum at 50°C to obtain 12.63 g of white solids with a yield of 90.1%.
[0027] Step 2) In a 1000ml four-necked flask, 12.63g of the product of Step 1), dissolve it in 300ml of methanol, slowly add 500ml of 0.05% sulfuric acid-methanol solution at room temperature, add 13.44g of potassium hydrogen persulfate after the addition, protect from light After reacting for 7 hours, the reaction solution was extracted with ...
Embodiment 2
[0032] Step 1) in N 2 Under protection, 16g of oleanolic acid is dissolved in a mixed solvent of 300ml of acetone and 20ml of DMF in a 1000ml four-necked bottle. 48ml of Jones reagent is slowly added dropwise at 0°C. After the dropwise addition, it is raised to room temperature and reacted for 3 hours; monitor the completion of the reaction and cool down To 0°C, slowly add 320ml isopropanol dropwise. After the dripping was completed, the reaction system was poured into ice water to precipitate solids, washed repeatedly with water, filtered with suction, and dried under vacuum at 50°C to obtain 13.52 g of white solids with a yield of 84.5%.
[0033] Step 2) In a 1000ml four-necked flask, 13.52g of the product of step 1), dissolve it in methanol, slowly add 406ml of 0.05% sulfuric acid-methanol solution at room temperature, add 10.8g of potassium hydrogen persulfate after the addition is complete, and react in the dark After 5 hours, the reaction solution was extracted with dichlor...
Embodiment 3
[0037] Step 1) in N 2 Under protection, 14g of oleanolic acid is dissolved in a mixed solvent of 280ml of acetone and 140ml of DMF in a 1000ml four-necked flask, 70ml of Jones reagent is slowly added dropwise at 0°C, after the dropwise addition, warm up to room temperature and react for 2 hours; monitor the completion of the reaction and cool down To 0°C, slowly drop 490ml of isopropanol. After the addition was completed, the reaction system was poured into ice water to precipitate solids, washed repeatedly with suction and filtered, and dried under vacuum at 50°C to obtain 11.74 g of white solids, with a yield of 83.87.
[0038] Step 2) In a 1000ml four-necked flask, 11.74g of the product of Step 1), dissolve in methanol, slowly add 587ml of 0.05% sulfuric acid-methanol solution at room temperature, add 15.26g of potassium hydrogen persulfate after the addition is complete, and react in the dark After 8 hours, the reaction solution was extracted with dichloromethane and dried at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com