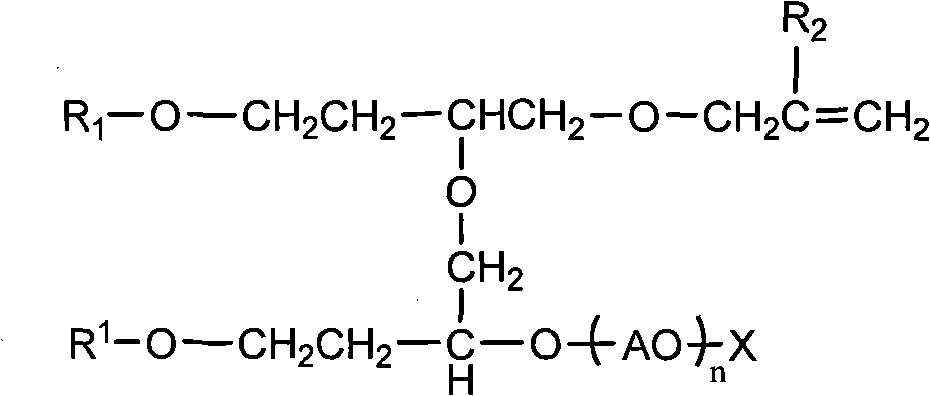
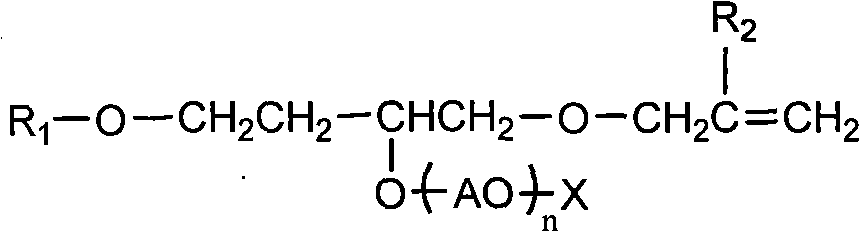Polymerizable surfactant
A technology of surfactants and polymerized monomers, used in the field of polymerizable surfactants, can solve the problems of poor emulsifying and dispersing properties, unenvironmental production processes, and high raw material prices, achieve excellent water resistance, improve mechanical stability and polymerization. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In the reactor with thermometer, stirrer, reflux tube, add 62 grams of allyl alcohol, 0.3 gram of sodium hydroxide as catalyst, then adopt dropwise to add 189 gram of isooctyl glycidyl ethers, temperature control is at 90- 100°C, the reaction time was 8 hours, and then the temperature was raised to 110°C, and excess allyl alcohol was removed under reduced pressure.
[0037] A total of 247 grams of the product of the above reaction was added to the epoxy addition kettle at a temperature of about 130°C and a pressure of 2-3kg / cm 2 , and ethylene oxide for addition reaction, the addition number is 5.
[0038] 467 grams of epoxy adducts and 97 grams of sulfamic acid were sulfonated at 120° C. for 1-5 hours to obtain product A, wherein the dimer content was about 2.1%.
Embodiment 2
[0040] Same as Example 1, except that 10 moles of ethylene oxide was added during epoxy addition to finally obtain product B, wherein the dimer content was about 2.0%.
Embodiment 3
[0042] Same as Example 1, only the amount of isooctyl glycidyl ether increases to 195 grams during the first step reaction, and the product obtained by the reaction adds 5 moles of oxirane equally, and then reacts with sulfamic acid to obtain product C, wherein The dimer content is about 6.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com