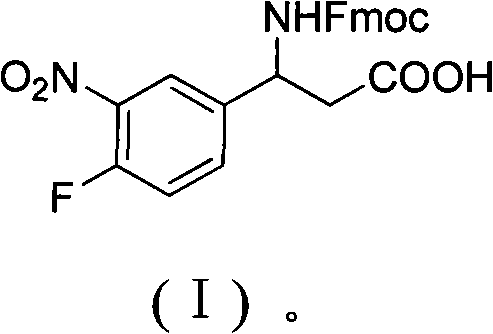
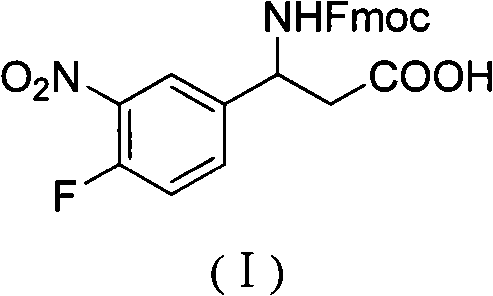
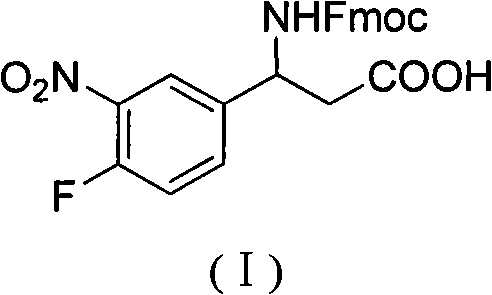
3-Fmoc amino-3-(3-nitro-4-fluophenyl) propionic acid and preparation method thereof
A kind of technology of fluorophenyl and nitro group, applied in the field of 3-Fmoc amino-3-propionic acid and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 18 grams of 4-fluorobenzaldehyde (15.6 milliliters, 0.145 moles, 1 equivalent, compound 4) was slowly added dropwise to 72 milliliters of concentrated sulfuric acid (commercially available concentrated sulfuric acid, concentration 98%) and 10.8 In the mixed solution of milliliter concentrated nitric acid (commercially available concentrated nitric acid, concentration is 68%). The dropping rate was 2 drops of 4-fluorobenzaldehyde / sec. After the dropwise addition, the reaction system was gradually raised to room temperature. After reacting at room temperature for 1 hour, the reaction system was poured into 450 g of ice cubes, and a large amount of solids were generated. After the ice cubes completely melted into water, the reaction system was filtered with suction to obtain a solid product. The aqueous phase was extracted twice with 250 ml of dichloromethane, and the solid product obtained by suction filtration was dissolved in 100 ml of dichloromethane. All organic ph...
Embodiment 2
[0040] 62 g of 4-fluorobenzaldehyde (53.7 ml, 0.5 mol, 1 equivalent, compound 4) was slowly added dropwise to a mixed solution of 186 ml of concentrated sulfuric acid and 18.6 ml of concentrated nitric acid at -5°C to 0°C. The dropping rate was 3 drops of 4-fluorobenzaldehyde / sec. After the dropwise addition, the reaction system was gradually raised to room temperature. After reacting at room temperature for 2 hours, the reaction system was poured into 1395 g of ice cubes, and a large amount of solids were generated. After the ice cubes completely melted into water, the reaction system was filtered with suction to obtain a solid product. The aqueous phase was extracted three times with 480 ml of ethyl acetate, and the solid product obtained by suction filtration was dissolved in 300 ml of ethyl acetate. All organic phases were combined, washed with saturated sodium bicarbonate solution until the pH value was 7-8, then washed with 350 ml of saturated brine, and then dried wit...
Embodiment 3
[0048] 93 g of 4-fluorobenzaldehyde (80.6 ml, 0.75 mol, 1 equivalent, compound 4) was slowly added dropwise to a mixed solution of 186 ml of concentrated sulfuric acid and 18.6 ml of concentrated nitric acid at -5°C to 0°C. The dropping rate was 4 drops of 4-fluorobenzaldehyde / sec. After the dropwise addition, the reaction system was gradually raised to room temperature. After reacting at room temperature for 2 hours, the reaction system was poured into 1860 g of ice cubes, and a large amount of solids were generated. After the ice cubes completely melted into water, the reaction system was filtered with suction to obtain a solid product. The aqueous phase was extracted twice with 826 ml of methyl tert-butyl ether, and the solid product obtained by suction filtration was dissolved in 400 ml of methyl tert-butyl ether. All organic phases were combined, washed with saturated sodium bicarbonate solution until the pH value was 7-8, then washed with 615 ml of saturated brine, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com