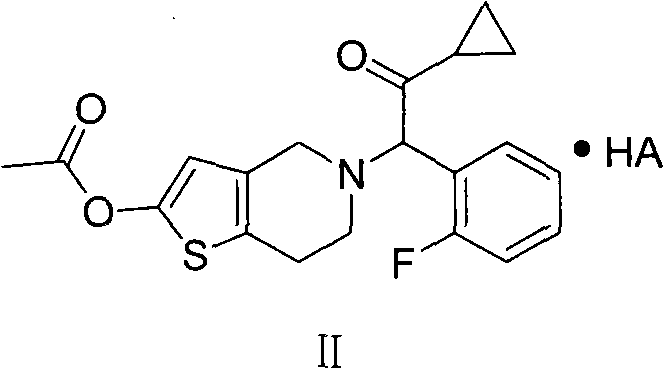
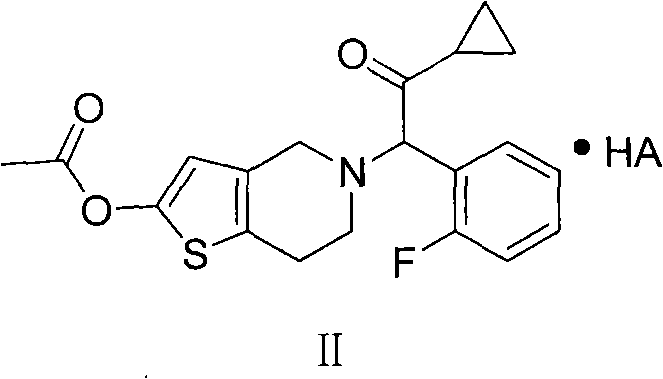
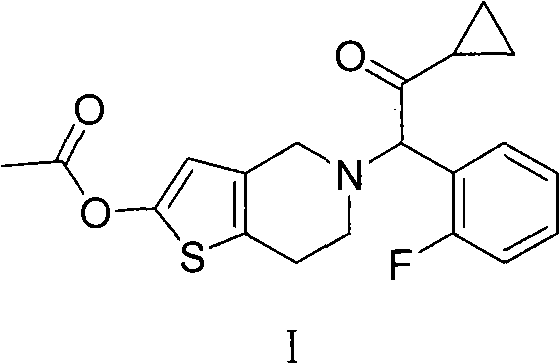
Prasugrel salt and preparation method thereof
A thunder salt compound, a Chinese-style technology, applied in the field of platelet inhibitor prasugrel salt and its preparation, can solve the problems of properties such as solubility, heat stability, moisture absorption, no detailed evaluation, and no obvious medicinal properties Advantages and other issues, to achieve the effect of good water solubility, good thermal stability and low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Prasugrel cystine salt
[0035] Dissolve 3.73g of prasugrel base in an appropriate amount of acetone / methanol (volume ratio 1 / 1), add dropwise the aforementioned organic solution of 2.52g of cystine, heat to reflux for 2h, filter after cooling to obtain prasugrel cystine salt Crude. Recrystallize from ethyl acetate to obtain 4.9 g of fine prasugrel cystine salt. Melting point: 250°C (carbonization).
Embodiment 2
[0036] Example 2: Prasugrel Phenylalanine Salt
[0037] Dissolve 3.73g of prasugrel base in an appropriate amount of acetone / methanol (volume ratio 1 / 1), add dropwise the aforementioned organic solution of 1.73g of phenylalanine, heat to reflux for 2h, filter after cooling to obtain prasugrel phenylalanine Crude acid salt. Recrystallized from ethyl acetate to obtain 4.19 g of fine prasugrel phenylalanine salt. Melting point: 250°C (carbonization).
Embodiment 3
[0038] Example 3: Prasugrel Threonine Salt
[0039] Dissolve 3.73g of prasugrel base in an appropriate amount of acetone / methanol (volume ratio 1 / 1), add dropwise the aforementioned organic solution of 1.25g of threonine, heat to reflux for 2h, filter after cooling to obtain prasugrel threonine salt Crude. Recrystallized from ethyl acetate to obtain 3.44 g of fine prasugrel threonine salt. Melting point: 250°C (carbonization).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com