White spot syndrome virus VP292 polypeptide and application thereof
A technology for prawns and recombinant proteins, applied in the field of genetic bioengineering, can solve problems such as difficult to eliminate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Cloning and sequencing of embodiment 1WSV254 gene fragment
[0045] Using WSSV genomic DNA as a template, primers P1 and P2 were used to amplify partial fragments of WSV237 gene.
[0046] P1: 5'-TTA CTCGAG ATGTTGTTTGATTTCT-3;
[0047] P2: 5'GAC AAGCTT TAATACGGGACCT-3';
[0048] The underlined part CTCGAG is the Xhol restriction site, AAGCTT is the HindIII restriction site,
[0049] The PCR reaction conditions are:
[0050] 94°C 10 minutes
[0051] 94°C for 30 seconds
[0052] 44.2°C for 30 seconds
[0053] 72°C for 1 minute (8 cycles)
[0054] 94°C for 30 seconds
[0055] 51.7°C for 30 seconds
[0056] 72°C for 1 minute (30 cycles)
[0057] 72°C 10 minutes
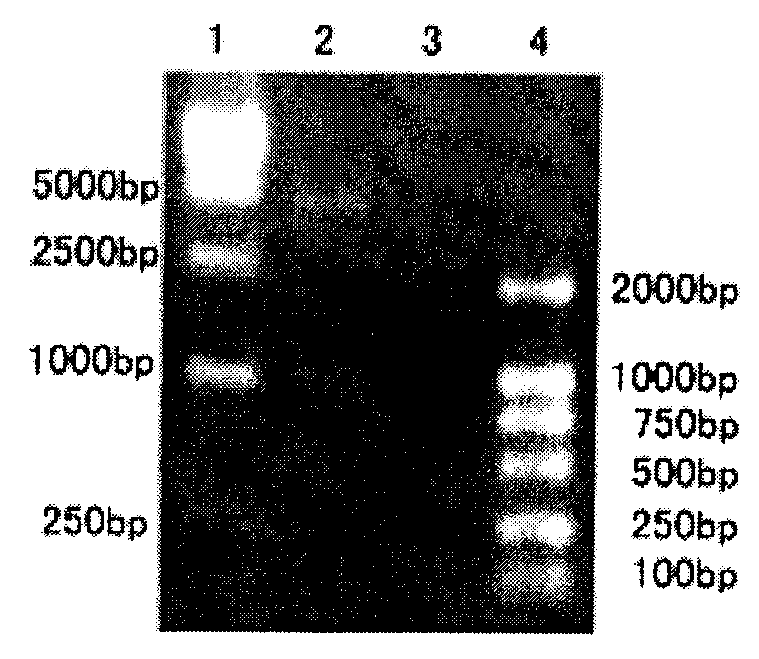
[0058] After the amplified fragment was purified by agarose gel electrophoresis, it was cloned into the prokaryotic expression plasmid vector pBAD / gIIIA to obtain the recombinant expression plasmid pBAD / gIIIA-rVP292, and then sequenced. The partial gene sequence of WSV237 obtained by sequencing is show...
Embodiment 2
[0060] Example 2 Expression and Purification of Recombinant VP292 Fragment
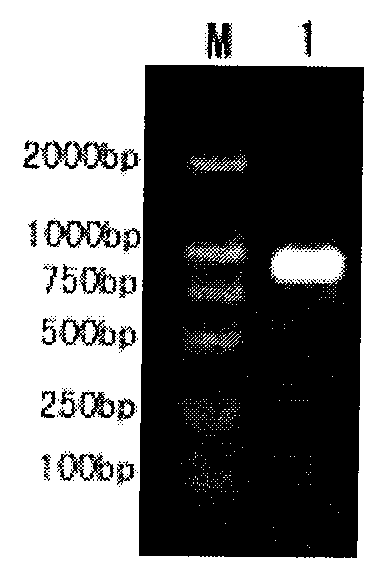
[0061] Transform the recombinant expression plasmid pBAD / gIIIA-VP292 containing the WSV237 gene into Escherichia coli TOP10, select positive clones and culture them in LB medium containing 100 mg / L ampicillin at 37°C until OD 600 When = 0.6, L-arabinose was added to a final concentration of 0.2%, and the cells were collected after induction at 37°C for 5 hours. Add ice-cold lysis buffer (1×PBS, 10mM NaHPO 4 , 140mM NaCl, 2.7mM KCL, 1.8mM KH 2 HPO 4 ), ultrasonically lyse the bacteria (300W×10s×10 times), centrifuge at 15,000rpm at 4°C for 20min, install on a Ni-nitriloacetic acid resin, wash with 5-10 column bed volumes of washing buffer (1×PBS) Remove impurities; elution buffer (50mM Tris-HCL, 100mM, imidazole, pH 8.0) elutes the target protein. The molecular weight of the purified protein was identified by SDS-PAGE to be about 12.09kD, and the purity was over 90%. see results figure 1.
Embodiment 3
[0062] Example 3 Antibody Preparation of Recombinant VP292 Fragment
[0063] The purified recombinant protein obtained in Example 2 was emulsified with an equal volume of complete Freund's adjuvant, and the mice were subcutaneously injected with 0.25-0.5 mg / mL emulsified protein, each 0.2 mL. Two weeks later, the same dose of the same antigen emulsified with incomplete Freund's adjuvant was re-injected to boost immunization to produce antibodies, and then boosted immunization every 10 days, at least twice. The titer and specificity of the obtained antisera were analyzed.
[0064] After reading the above content of the present invention, those skilled in the art can make various changes and modifications to the present invention, and these equivalent forms also fall within the scope defined by the appended claims of the present application.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com